Metabolic alkalosis
Notes
Overview
Metabolic alkalosis is characterised by a pH > 7.45 and raised plasma bicarbonate level (> 26 mmol/L).
There are numerous causes of metabolic alkalosis, which are broadly divided based on the volume status (i.e. fluid replete or replete) of the patient. It is characterised by alkalosis (pH > 7.45) and a high plasma bicarbonate level (> 26 mmol/L).
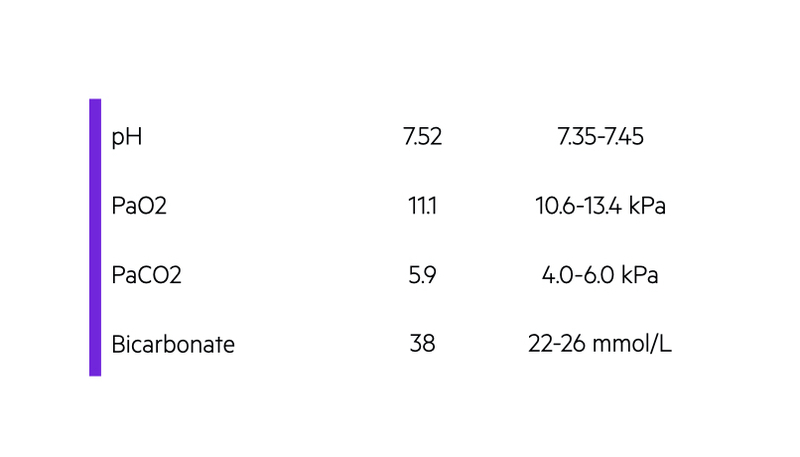
For metabolic alkalosis to develop, there needs to be an ‘initiating event' and then ‘maintenance of alkalosis’. In some cases, these two factors are the same physiological process.
Initiating event
Metabolic alkalosis, put simply, can result from a loss of hydrogen ions or gain of bicarbonate.
- Hydrogen ion loss: usually through the renal tract (e.g. diuretics) or GI tract (e.g. vomiting). Can also develop due to intracellular shifts of hydrogen ions.
- Gain of bicarbonate: alkalosis develops from exogenous administration of bicarbonate (e.g. sodium bicarbonate) or substances that can be broken down to bicarbonate (e.g. citrate within blood transfusion). Alternatively, endogenous metabolism may lead to formation of bicarbonate.
Maintenance
For development of metabolic alkalosis, the kidneys ability to regulate bicarbonate through excretion needs to be interrupted. Factors that interrupt bicarbonate excretion include:
- Chloride depletion: most common. Usually from vomiting and stomach acid loss or diuretics (e.g. loop or thiazide). Chloride depletion enhances bicarbonate reabsorption.
- Potassium depletion: multiple mechanisms. Causes intracellular shifts of hydrogen ions that enhances bicarbonate reabsorption. Enhances hydrogen ion secretion in tubules in exchange for potassium (net bicarbonate gain). Effects ammonium metabolism that generates bicarbonate. Finally, impairs chloride ion reabsorption in kidneys, which enhances hydrogen ion secretion (net bicarbonate gain).
- Reduced glomerular filtration: the renin-angiotensin-aldosterone system (RAAS) is activated by volume depletion or reductive effective circulating volume (e.g. heart failure, cirrhosis). RAAS enhances distal tubular sodium reabsorption, which leads to increased hydrogen ion and potassium losses. Hydrogen ion loss is matched by bicarbonate reabsorption.
Aetiology and work-up
The causes of metabolic alkalosis are numerous and broadly divided into chloride-responsive and chloride-resistant.
There are numerous causes of metabolic alkalosis. These are broadly divided based on the volume status of the patient.
- Volume deplete - also known as chloride-responsive (i.e. should respond to intravenous sodium chloride)
- Volume replete - also known as chloride-resistant (i.e. will not respond to intravenous sodium chloride)
The most common causes of metabolic alkalosis are GI losses from vomiting or diuretic therapy. This is usually obvious from the history and examination. However, a series of further investigations may be required to determine the underlying cause.
Urinary chloride
This is often used to help differentiate between chloride-responsive and resistance causes.
- Urinary chloride > 20 mmol/L: suggests chloride-resistant cases.
- Urinary chloride < 20 mmol/L: suggests of chloride-responsive cases.
Patients may be given volume expansion with normal saline empirically to see whether metabolic alkalosis will improve.
RAAS assessment
Some of the major causes of chloride-resistant metabolic alkalosis are due to overactivation of the RAAS system. Therefore, assessment of blood pressure, plasma renin and aldosterone are useful in differentiating the causes.
Additional investigations
Further tests may be required for chloride-resistant metabolic alkalosis and usually guided by the suspected aetiology. These include 24-hour urinary collections (e.g. aldosterone, cortisol), drug screen, adrenal imaging or dexamethasone suppression test.
Chloride-responsive metabolic alkalosis
The most common cause of chloride-responsive metabolic alkalosis is GI losses (e.g. vomiting).
- Gastric losses (severe vomiting)
- Colonic loss (inherited disorders, villous adenoma)
- Post diuretic therapy (loop/thiazide)
- Cystic fibrosis: loss of chloride in sweat
Chloride-resistant metabolic alkalosis
The causes of chloride-resistant metabolic alkalosis are broadly divided based on the presence or absence of hypertension.
With hypertension
- Hyperaldosteronism: primary (e.g. Conn’s syndrome), secondary (e.g. renin-secreting tumour)
- Pseudohyperaldosteronism (mimics hyperaldosteronism): liquorice ingestion, apparent mineralocorticoid excess (autosomal recessive inherited condition), Liddle’s syndrome (autosomal dominant inherited condition)
- Cushing’s syndrome
- Renal artery stenosis
Without hypertension
- Bartter's syndrome: autosomal recessive inherited disorder. Defect on think ascending limb of loop of Henle.
- Gitelman's syndrome: autosomal recessive inherited disorder. Defect on distal convoluted tubule.
- Current diuretic therapy (loop/thiazide)
- Profound potassium depletion
Other causes
There are several additional causes of metabolic alkalosis.
- Administration of bicarbonate (e.g. sodium bicarbonate therapy): usually requires presence of renal impairment to cause alkalosis.
- Milk-alkali syndrome: syndrome of hypercalcaemia, metabolic alkalosis and renal impairment. Due to excess intake of calcium carbonate, milk and/or antacids.
- Hypercalcaemia: volume depletion and enhanced bicarbonate reabsorption.
- Intravenous penicillin: due to affect of nonreabsorbable anions on renal sodium handling.
- Massive blood transfusion: citrate in blood products converted to bicarbonate.
- Refeeding: enhanced metabolism of ketoacids to bicarbonate.
Last updated: March 2021
Have comments about these notes? Leave us feedback
