Hyperthyroidism
Notes
Definition & classification
Hyperthyroidism is a common endocrine condition caused by an overactive thyroid gland causing an excess of thyroid hormone.
Hyperthyroidism affects around 1-2% of the population. It is important to first define the terminology:
- Hyperthyroidism: overactive thyroid gland (i.e. increased thyroid hormone production) causing an excess of thyroid hormone and thyrotoxicosis.
- Thyrotoxicosis: refers to an excess of thyroid hormone, having an overactive thyroid gland is not a prerequisite (e.g. ingestion of excess thyroid hormone).
Hyperthyroidism more commonly affects women than men (5-10 times more common) and may be associated with a number of autoimmune and endocrine conditions.
In this note, we focus predominantly on hyperthyroidism (thyrotoxicosis with hyperthyroidism) in adults, though we will briefly discuss thyrotoxicosis without hyperthyroidism.
Thyroid physiology
Thyroid hormone release is controlled by the hypothalamic-pituitary-thyroid axis.
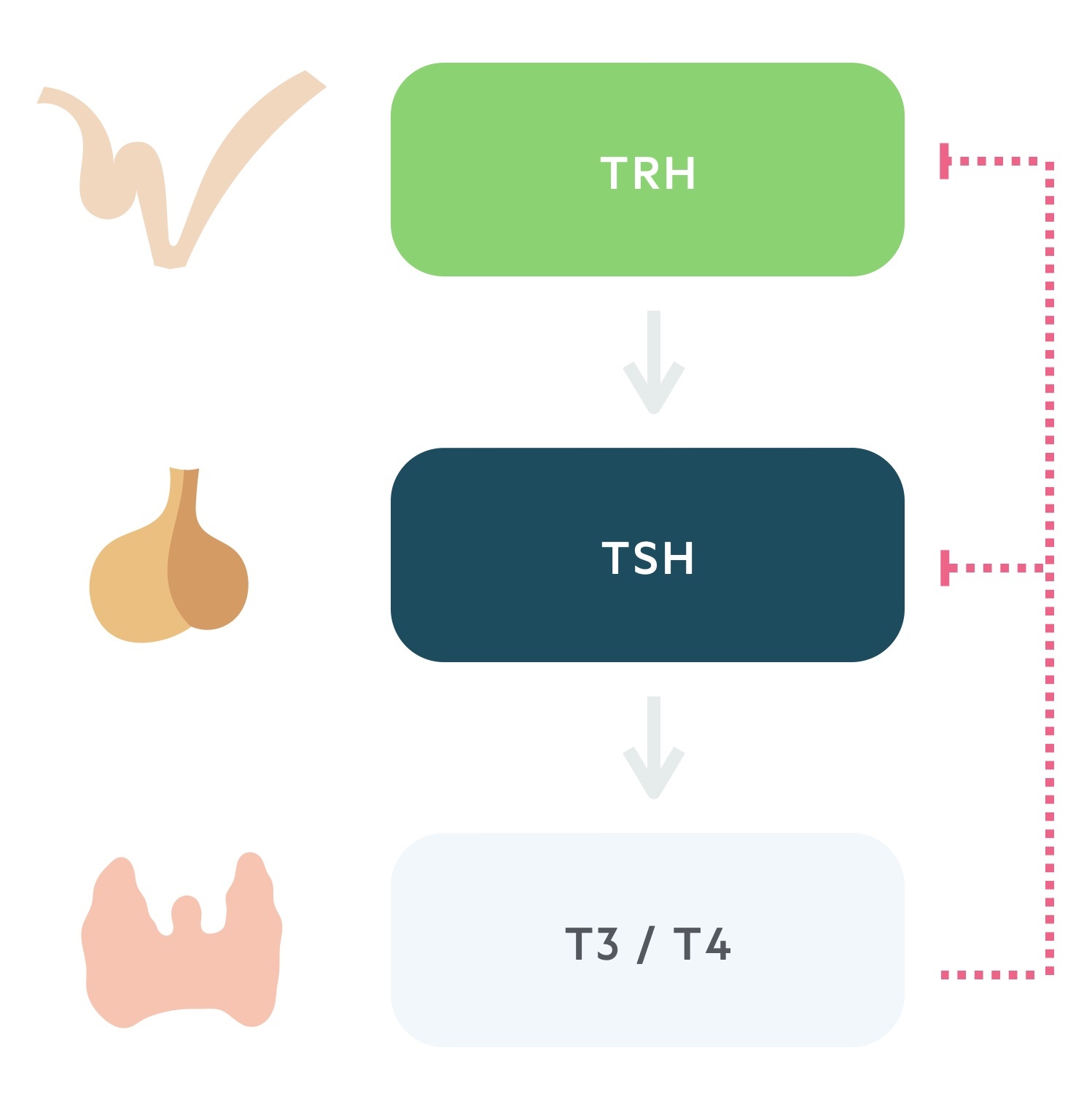
1. Thyrotropin releasing hormone
TRH is secreted from the paraventricular nucleus of the hypothalamus. As the name suggests it is a tropic hormone i.e one that acts upon another endocrine gland.
It reaches the anterior pituitary via the hypophyseal portal system. Here it causes the release of thyroid stimulating hormone.
2. Thyroid stimulating hormone
TSH, produced by the thyrotrophs of the anterior pituitary, is released following stimulation by TRH.
Transported in the blood, TSH acts upon the thyroid gland promoting the synthesis and release of thyroid hormone.
3. Triiodothyronine and thyroxine
The thyroid is stimulated to synthesise and release thyroid hormone by TSH. The thyroid produces two hormones, thyroxine (T4) and triiodothyronine (T3). These thyroid hormones complete a negative feedback loop through the suppression of TRH and TSH release.
Though T3 is more biologically active than its counterpart T4, secreted thyroid hormone is 90% T4. Peripherally much of T4 is converted to T3. Both are highly lipophilic, act on intracellular receptors and bind to thyroxine-binding globulin (TBG) in the blood. Only the ‘free pool’ is active: <0.1% T4 and <1% of T3.
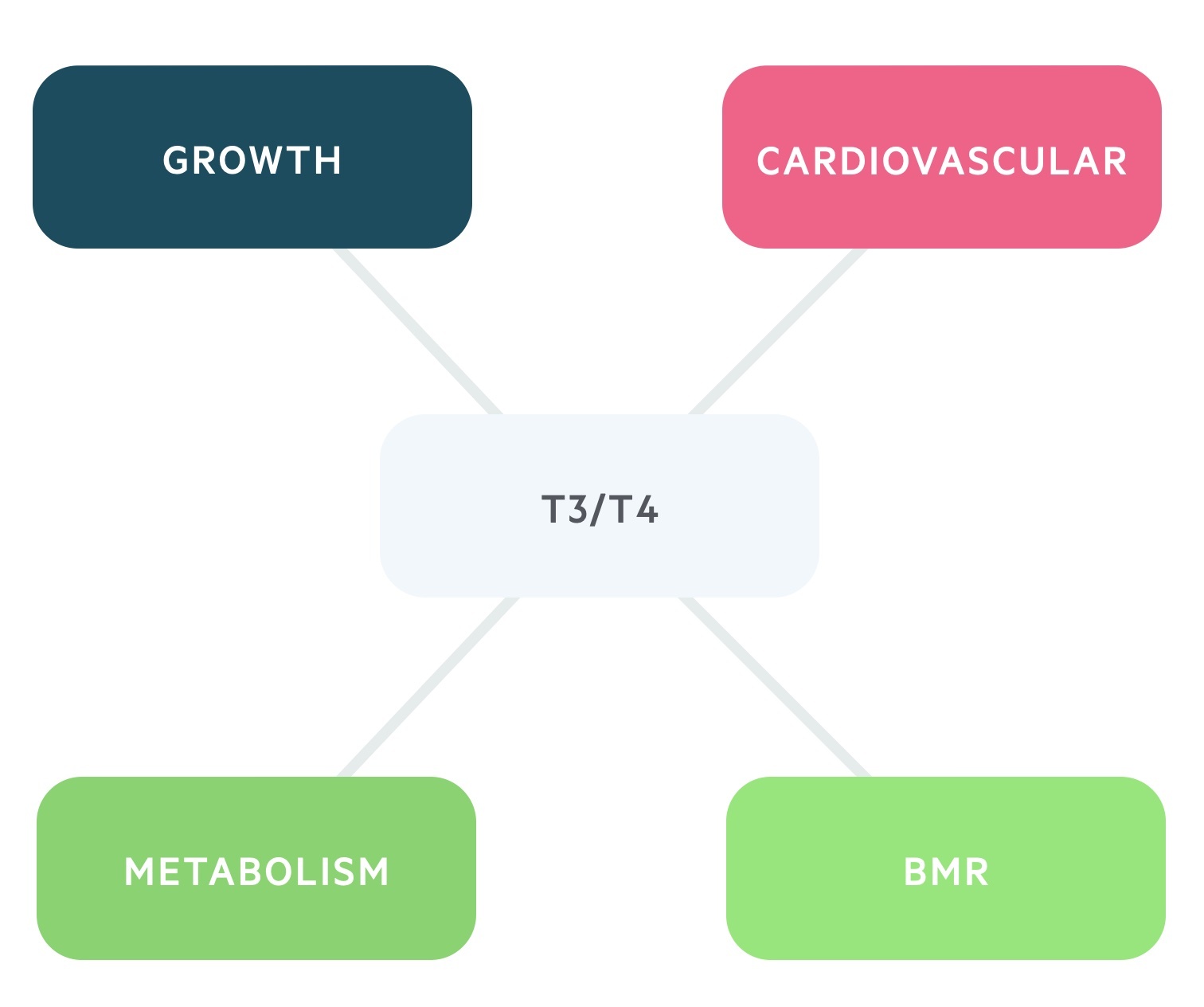
The effects to T3/T4 are numerous:
- BMR: increases the basal metabolic rate.
- Metabolism: it has anabolic effects at low serum levels and catabolic effects at higher levels.
- Growth: increases release and effect of GH and IGF-1.
- Cardiovascular: increases the heart rate and contractility through increasing sensitivity to catecholamines.
Thyrotoxicosis with hyperthyroidism
Thyrotoxicosis with hyperthyroidism is an excess of thyroid hormone caused by overactivity of the thyroid gland.
Graves’ disease
Graves’ disease is a common autoimmune condition and is the most common cause of hyperthyroidism (60-80% of cases) in the UK.
It is caused by IgG antibodies to the TSH receptors found within the thyroid. Termed thyroid-stimulating hormone receptor antibodies (TSHR-Ab), these antibodies mimic the action of TSH causing excessive stimulation of the gland.
Graves’ ophthalmopathy affects up to 30% of patients.
Toxic multinodular goitre
Toxic multinodular goitre is the second most common cause of hyperthyroidism in the UK.
In this condition, multiple autonomous nodules develop that are capable of producing and secreting thyroid hormones.
Solitary toxic adenoma
In this condition, a single adenoma develops and produces and releases excess thyroid hormones.
Amiodarone-induced thyrotoxicosis type 1
Amiodarone is a lipophilic class III anti-arrhythmic drug with a high iodine content. Its effects on the thyroid can be alarming and may cause both hypothyroidism and hyperthyroidism.
In type 1 the Jod-Basedow phenomenon, in which excess iodine intake causes excess thyroid hormone synthesis, occurs. It is seen in patients with pre-existing thyroid disease.
Follicular thyroid cancer
In metastatic follicular thyroid cancer, malignant tissue may remain functional. The increased amounts of tissue can lead to an overproduction of thyroid hormone.
Struma ovarii
This is a rare but interesting condition. Hyperthyroidism is caused by thyroid hormone release from ectopic thyroid tissue related to:
- Ovarian teratomas
- Dermoid tumours
The majority of these tumours are benign.
Beta-HCG related
Beta-HCG is thought to mimic the action of TSH causing thyroid hormone synthesis and release. It occurs in states of elevated Beta-HCG:
- Pregnancy
- Hydatidiform mole
- Choriocarcinoma
- Testicular germ cell tumour
Secondary hyperthyroidism
Secondary hyperthyroidism is characterised by excess TSH production stimulating increased T3/T4 to be produced and released. It is very rare and typically caused by a TSH-secreting pituitary adenoma.
Thyrotoxicosis without hyperthyroidism
Thyrotoxicosis may occur without hyperthyroidism (i.e. an overactive thyroid gland), these conditions are described as thyrotoxicosis without hyperthyroidism.
These conditions do not feature overactivity of the thyroid gland. This may be demonstrated by reduced or absent radioiodine uptake. It can result from:
- Thyroiditis: inflammation of the thyroid gland resulting in the release of stored thyroid hormone.
- Exogenous ingestion: a person ingests thyroid hormone.
Anti-thyroid drugs will have little effect in the majority of these conditions as increased synthesis of thyroid hormone is not always part of the aetiology.
Levothyroxine
When taken at supra-therapeutic doses levothyroxine may cause thyrotoxicosis without hyperthyroidism. Patients may abuse levothyroxine for weight-loss purposes.
De Quervain’s (subacute granulomatous) thyroiditis
This is a self-limiting condition, thought to be viral in origin. It results in inflammation of the thyroid gland and release of thyroid hormone.
It features three phases (though does not always follow this pattern):
- Thyrotoxicosis
- Hypothyroidism
- Resolution
It characteristically causes a painful goitre.
Amiodarone-induced thyrotoxicosis type II
Amiodarone is a lipophilic class III anti-arrhythmic drug with a high iodine content. Its effects on the thyroid can be alarming and may cause both hypothyroidism and hyperthyroidism.
Type 2 is caused by a destructive thyroiditis with resultant release of thyroid hormone.
Clinical features
Hyperthyroidism may be asymptomatic or present with non-specific malaise or signs of thyroid hormone excess.
Graves disease has a number of unique features that may be present which we discuss in the chapter below.
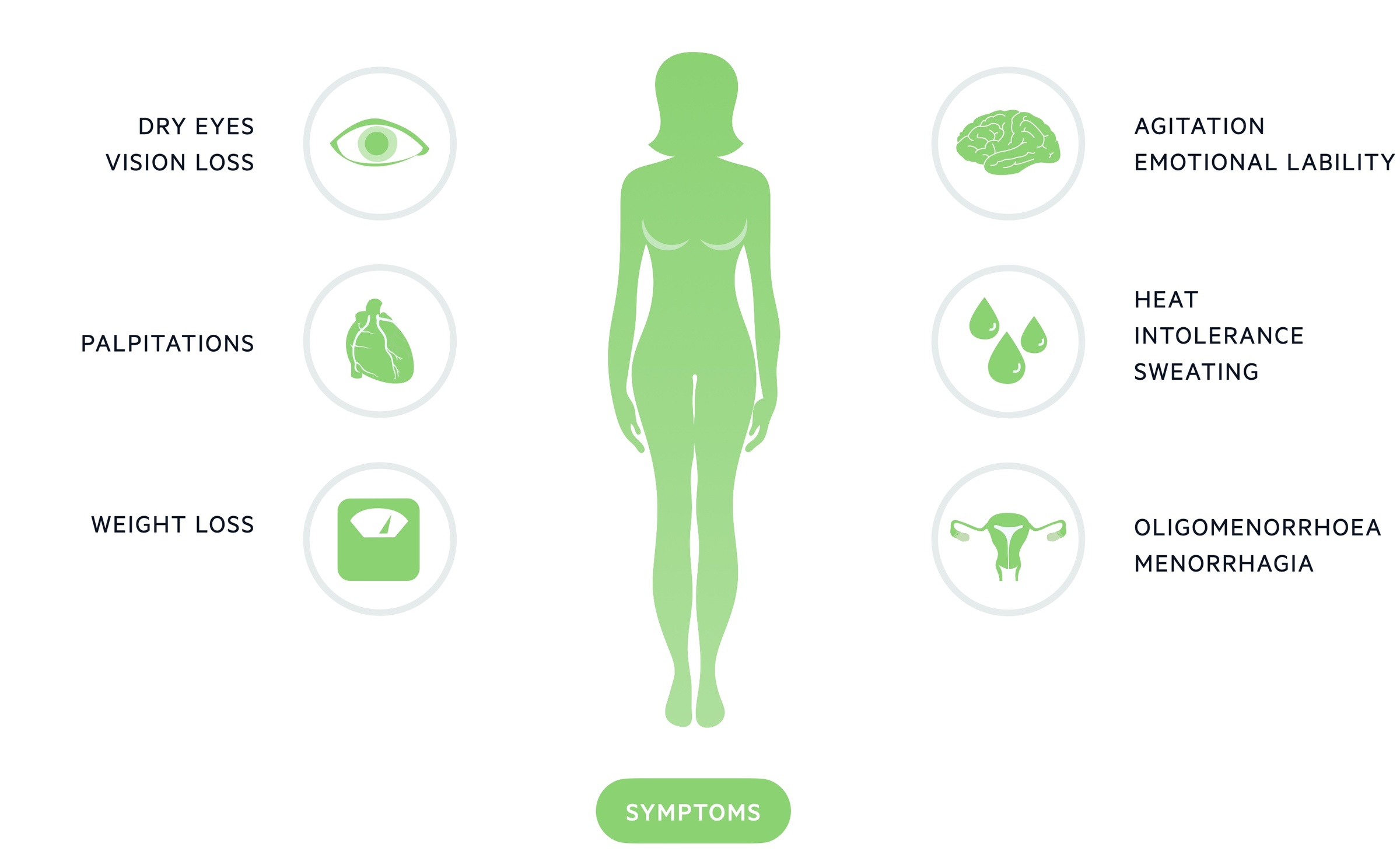
Symptoms
- Goitre
- Palpitations
- Heat intolerance
- Weight loss
- Diarrhoea
- Amenorrhoea
- Reduced libido
- Gynaecomastia (in men)
- Fatigue
- Change in mood
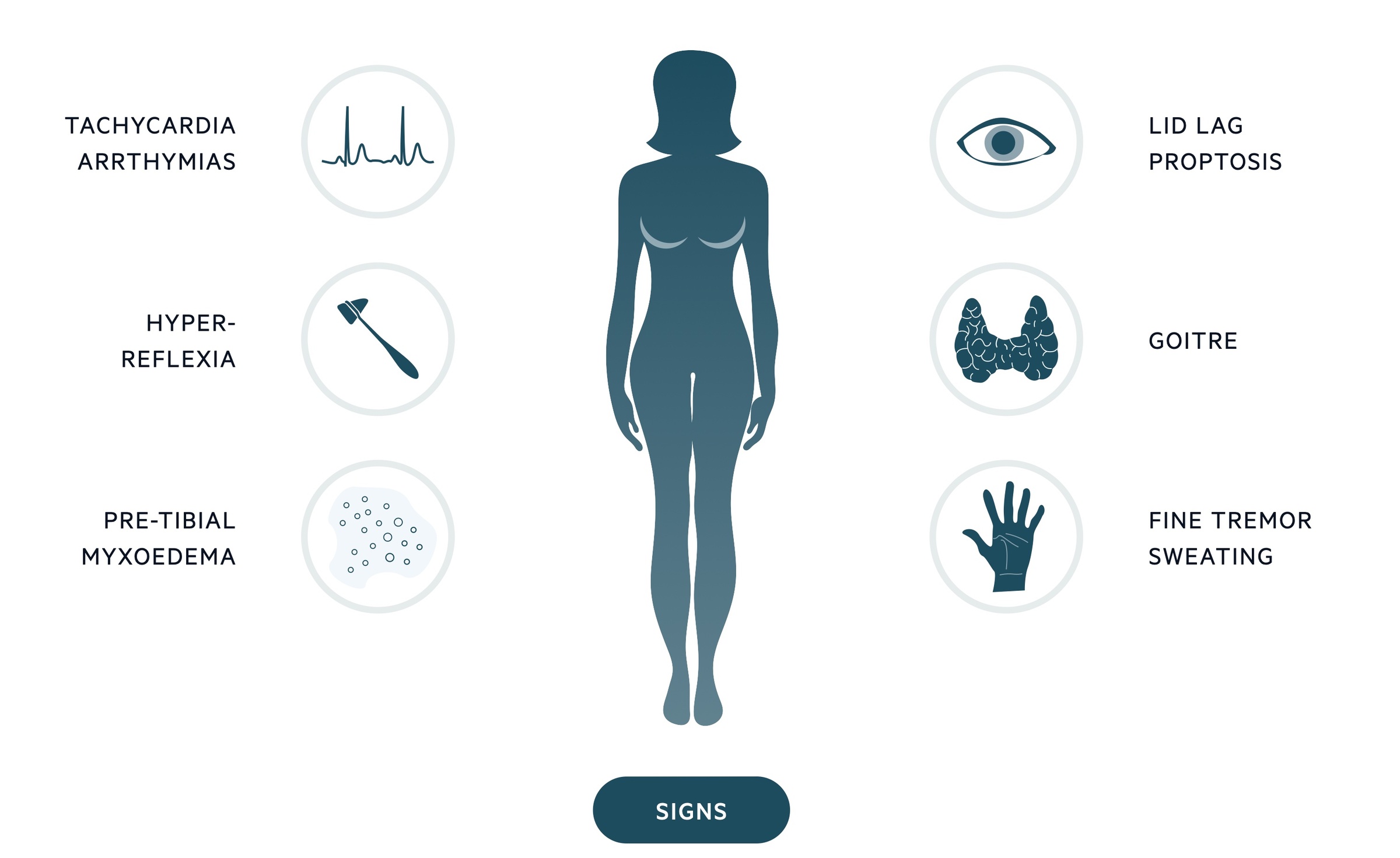
Signs
- Goitre
- Sinus tachycardia/arrhythmias
- Hair loss
- Palmar erythema
- Tremor
- Thyroid bruit (Graves’)
- Myxoedema - deposition of mucopolysaccharides in the skin leading to swelling.
Features of Graves’
Graves' disease has a number of unique features.
Graves’ ophthalmopathy
This condition affects up to 50% of patients with Graves', threatening sight in 5-10% of these cases. It is more commonly seen in smokers.
The pathogenesis of Graves’ ophthalmopathy is not fully understood, it is known that TSHR-Abs are involved. Inflammation and accumulation of mucopolysaccharides occur in the retro-orbital adipose tissue and extra-ocular muscles.
It may be classified with the American Thyroid Association system (using the mnemonic 'NOSPECS').
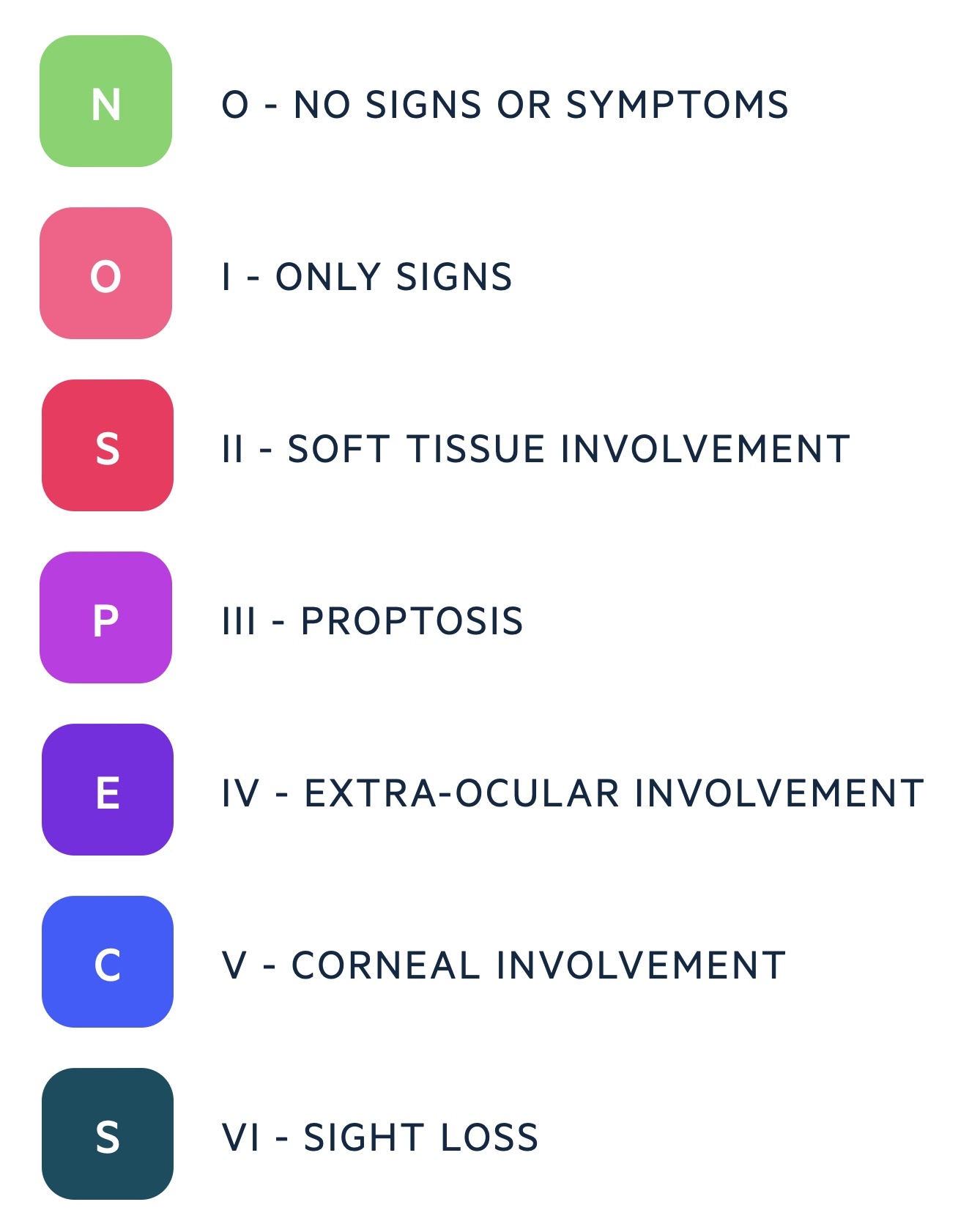
Onset may precede the presentation of hyperthyroidism or follow its treatment. Radioiodine is thought to increase the chances of developing Graves’ ophthalmopathy.
Features include proptosis, periorbital oedema, lid retraction, eye pain and visual loss.
Thyroid dermopathy
The most common manifestation is pretibial myxoedema. This is an infiltrative dermopathy resulting from deposition of mucopolysaccharides in the dermis.
Thyroid acropachy
This is characterised by periostitis, nail clubbing and soft tissue swelling of the extremities. It's most commonly seen in smokers with co-concomitant Graves’ ophthalmopathy.
Investigations & diagnosis
Measurement of TSH and fT4/fT3 are necessary to diagnose hyperthyroidism.
Hyperthyroidism should be suspected in anyone presenting with the suggestive features described above. In practice, clinicians should have a low threshold for ordering a thyroid profile as the symptoms are often non-specific.
NICE guidance (NG 145) also advise testing for thyroid dysfunction in those with:
- Type 1 diabetes
- Other autoimmune diseases
- New-onset atrial fibrillation
Testing should also be considered in those with mental health illnesses like depression and anxiety.
Thyroid profile
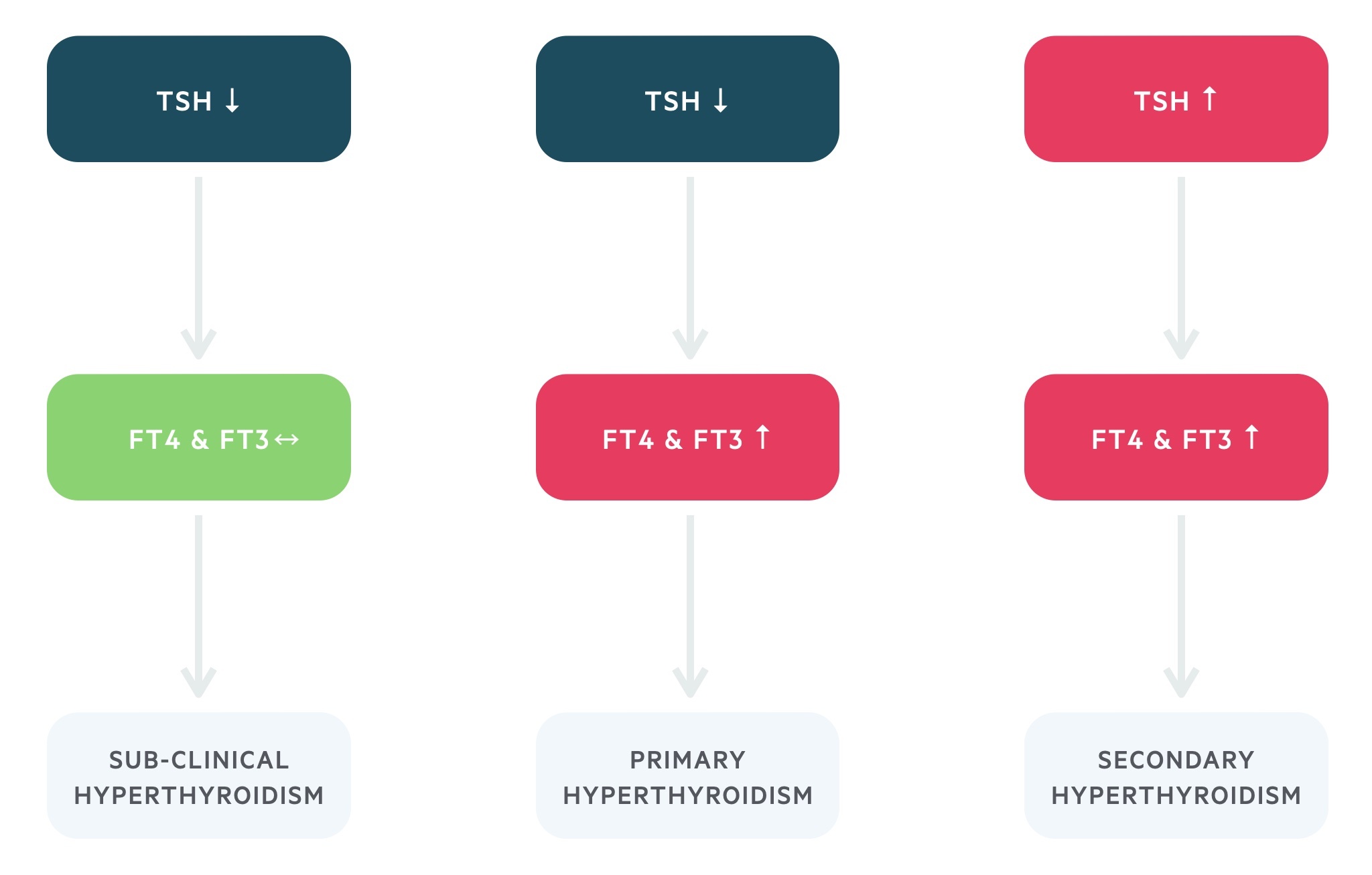
TSH levels are the single most important diagnostic test. You can consider sending for a TSH level alone when not suspecting secondary hyperthyroidism (i.e. pituitary tumour). If the TSH level is below the normal range free T4 (fT4) and free T3 (fT3) can be added on.
The thyroid profile will allow you to distinguish between subclinical, primary, and secondary disease:
- Primary hyperthyroidism: low TSH, raised fT4 and fT3
- Secondary hyperthyroidism (e.g. pituitary adenoma): high TSH, raised fT4 and fT3
The vast majority of cases of hyperthyroidism are primary in nature. In addition, there is a condition termed subclinical hyperthyroidism characterised by a low TSH with a normal fT4 and fT3. It is a complex condition whose treatment is still under debate. Ongoing monitoring is required in all cases as overt hyperthyroidism may occur.
Autoantibodies
Thyroid-stimulating hormone receptor antibodies (TSHR-Ab) are seen in > 90% of those with Graves’ disease.
Imaging
May be used if the aetiology is uncertain or malignancy suspected:
- Ultrasonography
- Thyroid uptake scan:
- Thyrotoxicosis with increased uptake: indicative of increased hormone synthesis
- Thyrotoxicosis with decreased uptake: indicative of inflammation/destruction of the thyroid gland with the release of thyroid hormone
Management
Anti-thyroid drugs (thioamides), radioactive iodine (RAI) and surgery are all viable management options.
Management is complex! Here we will outline the three main modalities, discussing, in particular, the management of Graves', toxic multinodular goitre and solitary toxic adenoma (i.e. causes of thyrotoxicosis with hyperthyroidism).
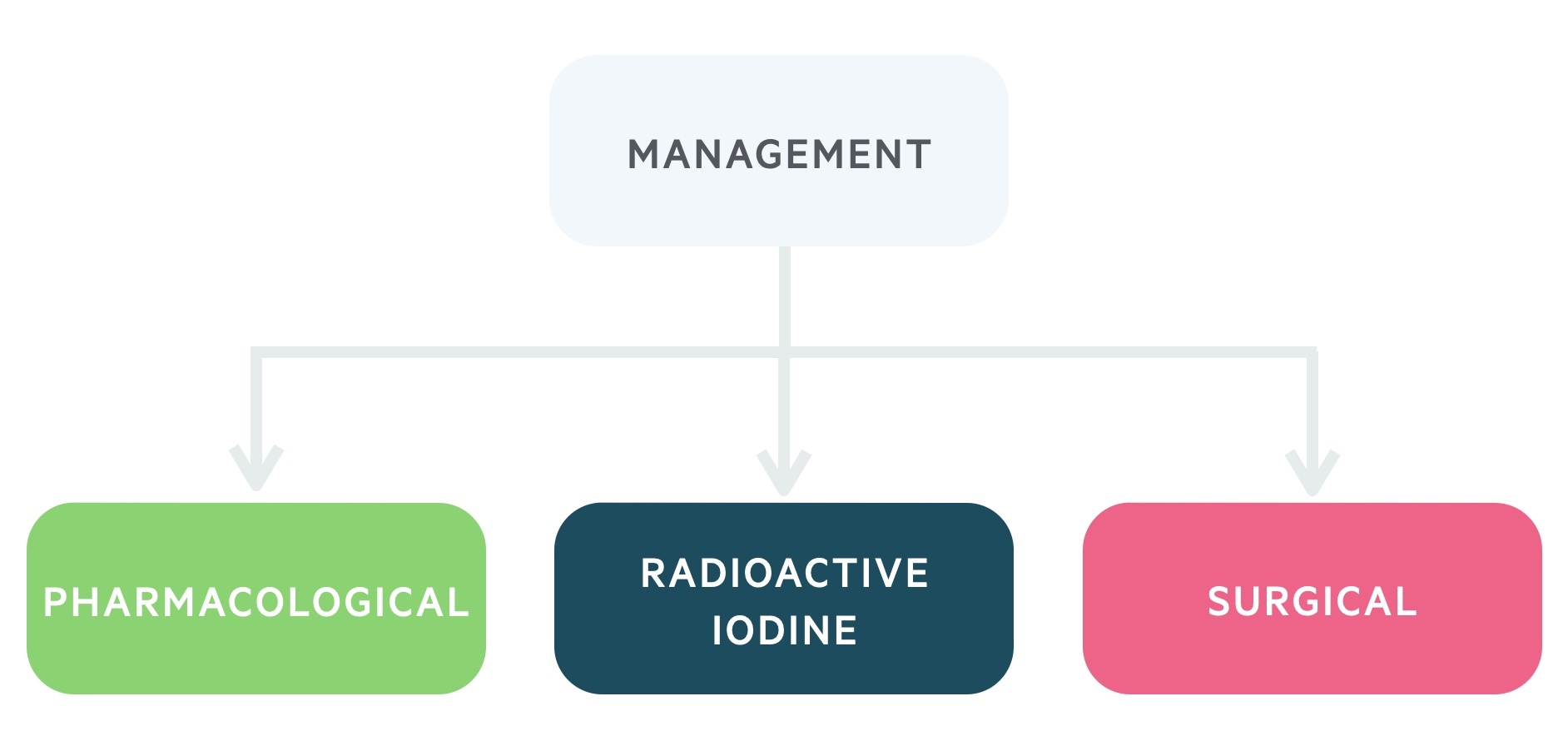
NICE guideline (NG145): Thyroid disease: assessment and management (published 2019, last accessed 2021) provide a comprehensive overview of the management of hyperthyroidism. Here we outline some of the key aspects of this guidance that you need to know as a student.
Initial therapy
Following diagnosis, patients are normally offered thioamides (carbimazole or propylthiouracil) whilst a decision on definitive therapy is being made. Definitive therapy may be radioactive iodine, ongoing thioamide therapy or surgery.
A beta-blocker or calcium channel blocker may be prescribed to treat adrenergic symptoms whilst the thioamides take effect.
Radioactive iodine
Iodine is key to the synthesis of thyroid hormones. As such when radioactive iodine (RAI) is given it is taken up by the thyroid causing destruction and reduced thyroid hormone release.
Due to the radioactive nature of the treatment, there are a number of contraindications:
- Current pregnancy
- Falling pregnant within 6 months of treatment
- Breastfeeding
- Fathering a child within 4-6 months of treatment
For a short time following treatment, the patient will emit some radiation. As such they will be advised to avoid close contact with members of their household and restrict any contact with children or pregnant women for several weeks.
It is typically offered first-line in Graves' in those suitable and not likely to enter remission. It is often offered as first-line definitive management for toxic multinodular goitre.
In Graves, RAI may exacerbate or cause ophthalmopathy.
Thioamides
The thioamides carbimazole or propylthiouracil may be used. These drugs reduce thyroid synthesis working over a number of weeks. May be offered first line in Graves' disease, particularly if likely to have remission (mild, uncomplicated disease). Remission may be seen after 18-24 months of treatment, demonstrated by a trial without therapy.
In the UK carbimazole is used first-line. It is teratogenic and subject to Medicines and Healthcare products Regulatory Agency guidance (found here) on safety in those with childbearing potential. It is associated with congenital malformations, particularly when taken in the first trimester. Those with childbearing potential must be aware of this risk and use effective contraception.
Propylthiouracil can be considered in adults where the patient is/has:
- Intolerant/allergic to carbimazole
- Pregnant or planning on pregnancy in the next 6 months
- A history of pancreatitis
Baseline FBC and LFTs are obtained prior to the commencement of thioamides. Neutropenia or severely deranged transaminases are a contraindication to treatment. Agranulocytosis is a severe side effect associated with both thioamides. It refers to an absence or reduction of granulocytes - in particular neutrophils. This neutropenia puts patients at risk of severe infection and sepsis.
Two regimes available:
- Block and replace: thioamides are given at a level sufficient to block endogenous T3/T4 production alongside levothyroxine. This regime must not be used in pregnancy.
- Dose titration: thioamides are given alone, dose adjusted to give normal levels of TSH.
In those with toxic nodules, treatment is likely life-long if chosen as definitive management (hence surgery or RAI is often preferred).
NOTE: The decision about whether to treat subclinical hyperthyroidism is complex. Some 5% of patients with subclinical disease will develop overt features each year. Decisions about treatment should take the underlying aetiology, severity and associated comorbidities into account.
Thyroidectomy
A thyroidectomy (removal of the thyroid gland) may be used as definitive therapy if malignancy is suspected, there is a compressive goitre or RAI/ anti-thyroid medications are unsuitable.
Hemithyroidectomy (removal of half the thyroid gland) may be offered to patients with a solitary toxic nodule.
Complications of the surgery include:
- Hypocalcaemia: affects 10%, typically transient, may be permanent in a minority of cases. Due to the proximity of the parathyroid glands to the thyroid gland.
- Recurrent laryngeal nerve injury: presents with hoarse voice post-operatively.
Graves’ ophthalmopathy
Management of Graves’ ophthalmopathy is dependent on disease severity.
In mild disease ('NO') artificial tears, elevation of the head during sleep and sunglasses may help symptoms. Smoking cessation is always advised.
In more severe disease ('SPECS') further management is necessary. Referral to an ophthalmologist is required if there is evidence of optic nerve compression, corneal opacity or inability to close an eye.
Treatment may include:
- Steroids
- Irradiation
- Surgical decompression
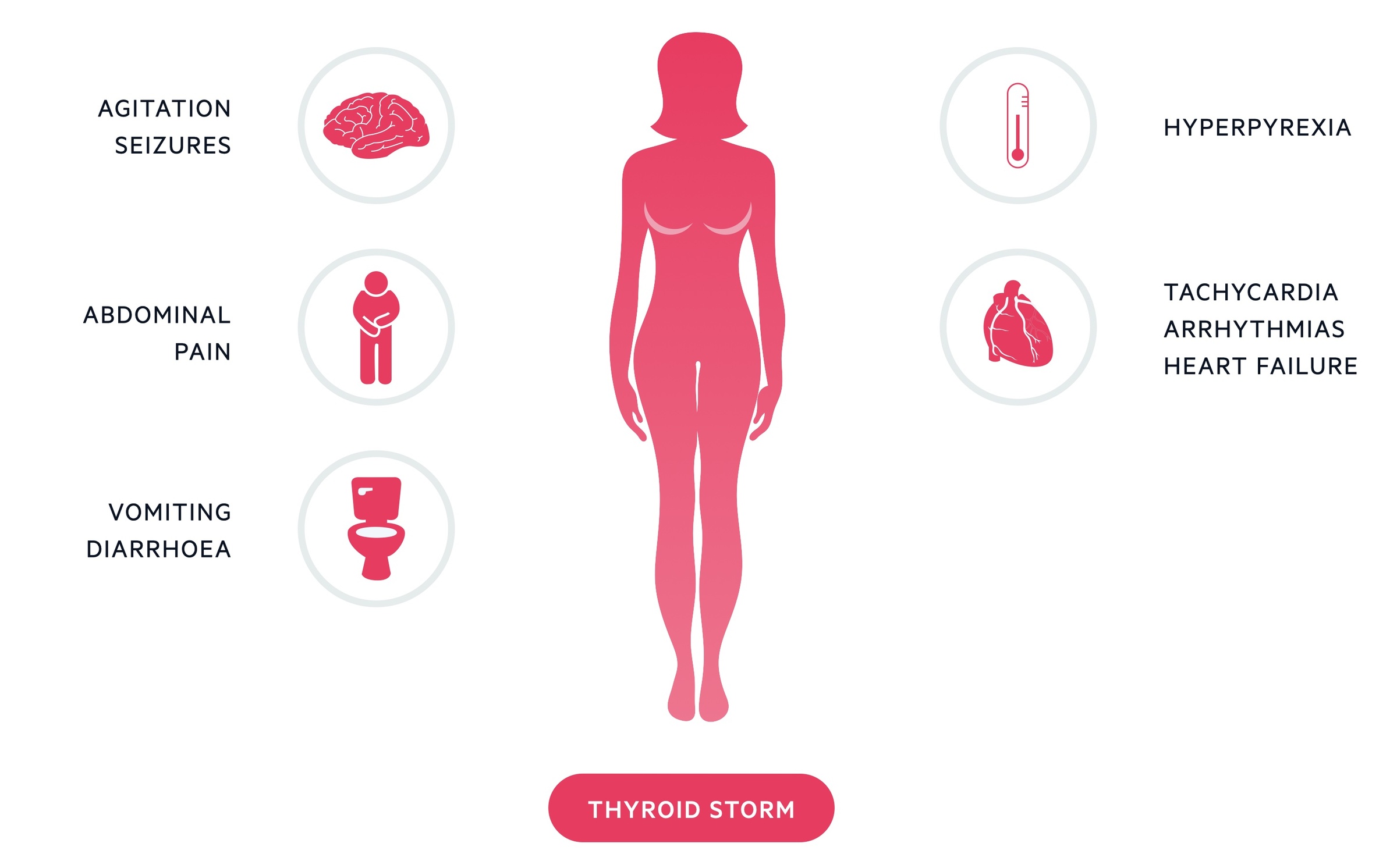
Thyrotoxic crisis
A thyrotoxic crisis is rare but potentially fatal result of untreated/undertreated hyperthyroidism.
It is a medical emergency that requires admission to hospital that may result from decompensation during an intercurrent illness.
It presents with hyperthermia, tachycardia, arrhythmias, nausea, vomiting, seizures and cognitive decline.
Management involves:
- Beta-blockers
- Thionamides: typically propylthiouracil, which in addition to its anti-thyroid effect also reduces the conversion of T4 to T3.
- Corticosteroids: reduce the conversion of T4 to T3.
- Lugol's iodine
Electrolyte imbalances, heart failure and hyperthermia should be addressed. Blood sugar must be monitored. Dialysis or plasma exchange may be required.
Last updated: November 2021
Have comments about these notes? Leave us feedback
