Barrett's oesophagus
Notes
Overview
Barrett’s oesophagus refers to columnar metaplasia of the distal oesophagus.
Barrett’s oesophagus (BO) is defined as any portion of the normal distal oesophageal squamous epithelial lining being replaced by metaplastic columnar epithelium. Metaplasia refers to the transformation one mature cell type into another.
This metaplastic change is the consequence of persistent oesophageal injury due to chronic reflux of stomach content. The condition is important because it can predispose to the development of oesophageal adenocarcinoma with a 0.33% annual risk in patients with BO without dysplasia (i.e. abnormal cells).
Epidemiology
Barrett’s oesophagus is a prevalent condition.
BO is estimated to occur in 5-15% of patients undergoing upper gastrointestinal endoscopy for reflux symptoms. It is even estimated to affect 0.9-10% of the general population.
The condition is more common in Caucasian males with a 2:1 male-to-female ratio.
Aetiology & pathophysiology
BO develops due to chronic oesophageal injury from chronic reflux of gastric content.
The lower oesophagus marks the junction between oesophagus and stomach at the GOJ. Tonic contractions of the lower oesophageal sphincter, coupled with extrinsic compression of the right crus of the diaphragm, are important to prevent acidic stomach content from entering the oesophageal lumen.
These mechanisms are not perfect and a small amount of reflux of stomach content (i.e. the refluxate) is common, but usually not associated with symptoms. Prolonged exposure to the refluxate can lead to symptomatic gastro-oesophageal reflux disease, oesophagitis and erosions. In addition, chronic damage can lead to transformation of normal squamous epithelium into metaplastic columnar epithelial cells. The identification of this metaplastic change is consistent with BO.
BO is a precursor to the development of adenocarcinoma, which may occur through further reflux-associated DNA damage, genetic alterations and uncontrolled cellular proliferation.
Risk factors
- Long standing gastro-oesophageal reflux
- Male sex (male-to-female ratio 2:1)
- Caucasian ethnicity
- Increasing age
- Obesity
- Smoking
- Family history
Transformation zone
Metaplasia is a term used to describe the transformation of one mature cell type into another. This type of cellular change is common around transformation zones where one cell type meets another.
Examples:
- Lower oesophagus: lower oesophageal squamous epithelium meets gastric columnar epithelium
- Cervix: normal ectocervix squamous epithelium meets the endocervical columnar epithelium at the external os.
Clinical features
Patients with BO usually have symptoms of chronic reflux, but the metaplastic columnar epithelium per se is asymptomatic.
Signs and symptoms
- Heartburn
- Regurgitation
- Chest discomfort
- Dyspepsia
- Nausea and/or vomiting
- Dysphagia (suggestive of stricture or malignancy in context of BO and reflux)
Approximately 40% of patents with BO will have no history of troublesome reflux.
Diagnosis
BO is an endoscopic diagnosis, which is subsequently confirmed on oesophageal biopsies.
The diagnosis of BO is made during upper gastrointestinal endoscopy by direct visualisation of the lower oesophagus.
The squamo-columnar junction, known as the ‘Z’ line, should lie within 1cm of the GOJ, which is marked by the top of the gastric folds. Therefore, if there is evidence of metaplastic columnar epithelium ≥1cm above the GOJ biopsies should be taken to confirm the diagnosis of BO.
Prague criteria
The Prague criteria refers to the endoscopic description of BO, which is divided into two components:
- Circumferential (C) extent: maximal circumferential height of BO
- Maximal (M) length: refers to the longest segment of BO
The length of BO is important. Length is independently associated with risk of cancer progression.
- Short segment Barrett's (< 3cm)
- Long segment Barrett's (> 3cm)
Histology
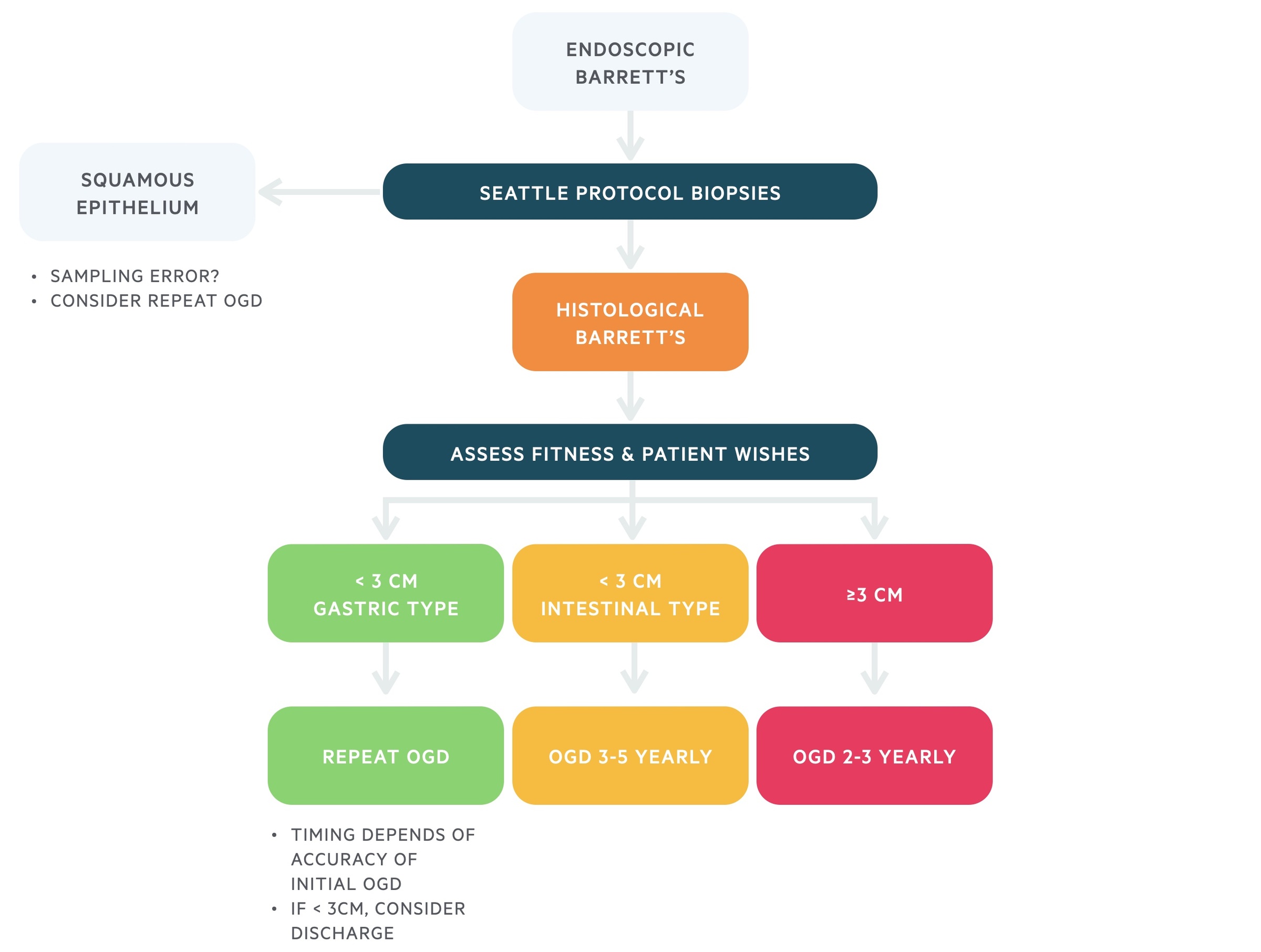
BO should be sampled using the Seattle protocol that follows four quadratic biopsies every 2cm, with additional biopsies of visible lesions. This is to look for any areas of dysplastic BO, which has a higher risk of cancer progression.
Biopsies can be sent for histological assessment to confirm the presence of metaplastic columnar epithelium and exclude the presence of dysplasia.
There are different types of histology that may be reported:
- BO with gastric/fundic metaplasia
- BO with intestinal metaplasia
The type of metaplasia and length of BO may influence surveillance regimens.
Management
The management of BO depends on the extent of disease and presence/absence of dysplasia.
Proton pump inhibitors
Proton pump inhibitors (PPIs) are recommended in all patients with BO. PPIs prevent acid production within the stomach through inhibition of H+/K+ ATPases in parietal cells. This reduces the acidity of the refluxate and therefore the damage to the lower oesophageal mucosa. PPI reduce risk of both high grade dysplasia and oesophageal carcinoma.
Surveillance
Patients with BO should be offered endoscopic surveillance. There are two surveillance regimens that depends on the presence or absence of dysplasia. The principle aim of surveillance is to detect high-grade dysplasia and/or oesophageal adenocarcinoma at a much earlier stage, which associated with improved survival.
Endoscopic therapy
Patients with confirmed low-grade or high-grade dysplasia may be offered endoscopic therapy. It is important to exclude oesophageal adenocarcinoma and further investigations may be needed (e.g. endoscopic ultrasound). These patients should be discussed at an upper gastrointestinal multidisciplinary meeting (MDM).
Surveillance
Endoscopic surveillance is recommended in patients with BO.
Patients with BO are at risk of oesophageal adenocarcinoma and as such require surveillance. The below recommendations are based on the British Society of Gastroenterology guidelines for the management of Barrett's oesphagus 2014 (including revised guidelines in 2017).
Non-dysplastic Barrett's
Following endoscopic identification of BO, patients will have biopsies to confirm the diagnosis. If dysplasia is identified on biopsy, management should follow dysplastic Barrett's surveillance. If no dysplasia is identified on biopsy, management should follow non-dysplastic surveillance.
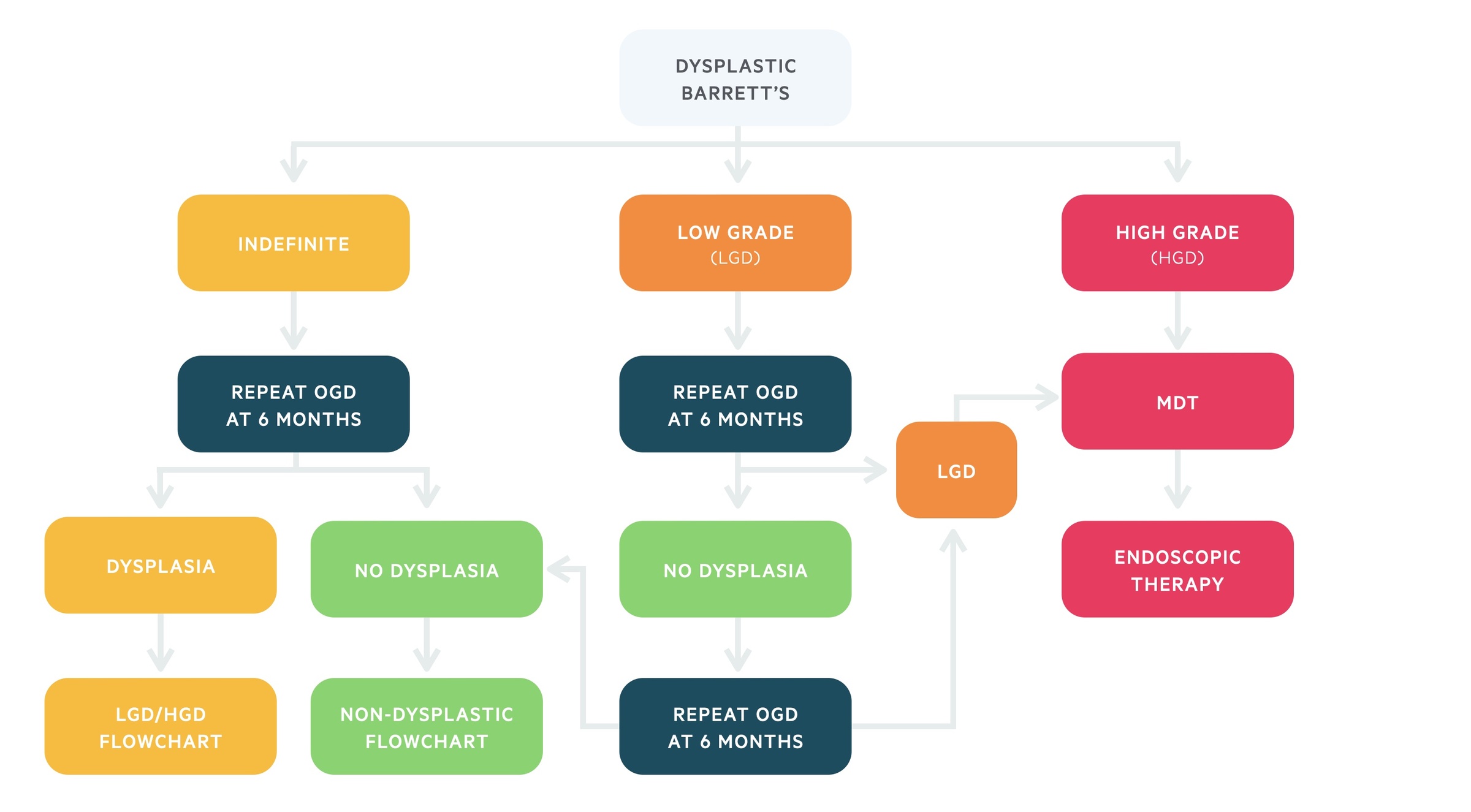
Dysplastic Barrett's
Patients with confirmed low-grade or high-grade dysplasia should be discussed at an upper GI MDM for consideration of endoscopic therapy. If dysplasia resolves, either with endoscopic therapy or PPI, and no dysplasia is found on repeat endoscopies then patients can be moved back to non-dysplastic surveillance.
Screening
Universal screening for BO is currently not recommended.
Currently, screening for BO is recommended only in patients with chronic gastro-oesophageal reflux disease and multiple risk factors (≥3) including male sex, Caucasian, >50 years old, personal/family history of BO or oesophageal cancer.
Currently, trials are ongoing to assess the validity of non-endoscopic devices and biomarkers to risk stratify low risk patients who do not need endoscopic assessment.
Last updated: March 2021
Have comments about these notes? Leave us feedback
