Meningitis
Notes
Overview
Meningitis refers to inflammation of the meninges, which are the outer membranes covering the brain and spinal cord.
Meningitis broadly refers to inflammation of the meninges, which may be due to a number of infectious and non-infective aetiologies. The most devastating of these is bacterial meningitis, which is a life-threatening condition and one of the major causes of infection-related deaths worldwide. In these notes, we predominantly focus on bacterial meningitis.
Epidemiology
The annual incidence of bacterial meningitis in developed countries is estimated at 2-5 per 100,000 population.
Further epidemiological data is dependent on the underlying aetiology. Meningitis has been reported to be ten times more common in developing countries due to less well developed preventative programmes (e.g. vaccination).
The median age of diagnosis of meningitis depends on the microorganism implicated (see chapter on aetiology).
Anatomy
The meninges are composed of three individual layers.
The meninges form the outer membranes covering of the brain and spinal cord. These are divided into three structures.
- Dura mater: tough outer membrane. Lies directly beneath the skull. Composed of two layers: outer periosteal layer and inner meningeal layer.
- Arachnoid mater: avascular layer of connective tissue that sits beneath the dura mater. Beneath the arachnoid mater is the subarachnoid space that contains cerebrospinal fluid.
- Pia mater: thin inner membrane. Tightly adherent to the brain and spinal cord.
Aetiology
Meningitis may be caused by a series of infectious and non-infectious aetiologies.
Infectious causes may be bacterial, viral, fungal or tuberculosis. Both bacterial and viral aetiologies are commonly implicated. Aseptic meningitis generally refers to meningitis where routine bacterial cultures are negative. This may be due to an incompletely treated bacterial infection, other infectious microorganisms or a non-infectious aetiology.
Non-infectious causes include malignancy, systemic inflammatory conditions (e.g. systemic lupus erythematous, Behçet's disease), head injury, medications (e.g. NSAIDs, co-trimoxazole) or surgery.
Bacterial
Bacterial meningitis usually occurs acutely (< 1 day) and can lead to profound sepsis and subsequent complications.
Commonly implicated organisms include:
- Neisseria meningitidis
- Streptococcus pneumoniae
- Haemophilus influenzae
- Listeria monocytogenes
- Escherichia coli
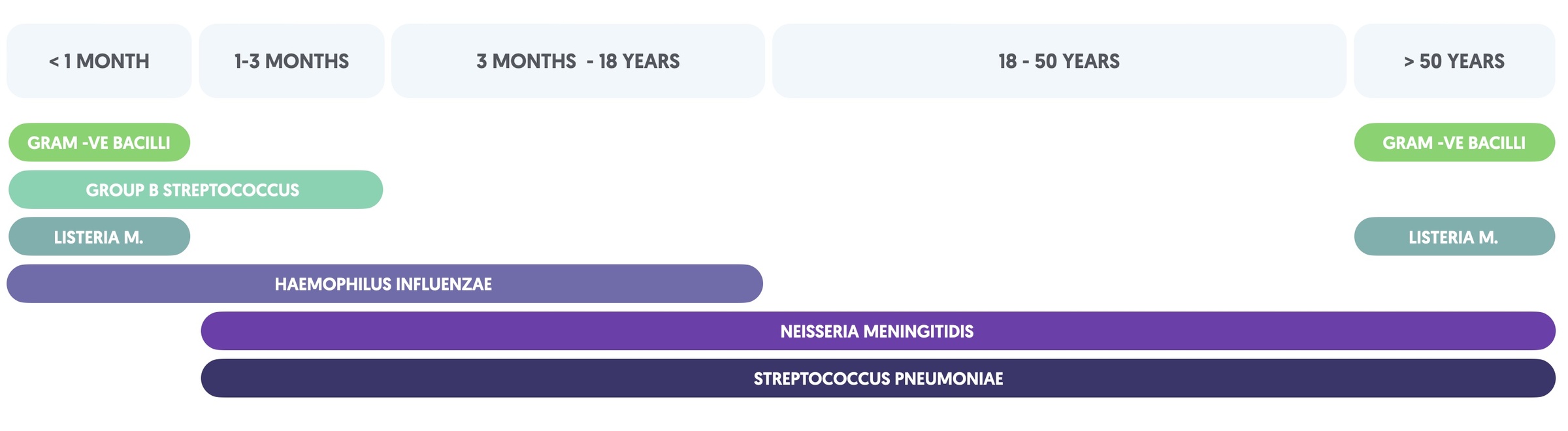
In the UK, N. meningitidis, S. pneumoniae and H. Influenzae account for the majority of bacterial meningitis in patients aged 3 months and older. The causative organism usually depends on the age and comorbidity of the patient. For example, patients with inherited complement deficiencies are at increased risk of Neisseria infections. In neonates, a broader range of organisms may be causative including Streptococcus agalactiase, gram negative organisms (e.g. E. coli) and L. monocytogenes.
The table summarises the likely organisms based on age.
Viral
Several viruses are implicated in meningitis
- Enteroviruses (most common): examples include echovirus and Coxsackieviruses
- Herpes simplex virus (HSV)
- Human immunodeficiency virus (HIV)
- West Nile virus (WNV)
- Varicella-zoster virus (VZV)
Viral meningitis is usually a less severe, self-limiting infection. It is important to differentiate viral meningitis from encephalitis, which presents with abnormal brain function (e.g. altered mental status, motor/sensory deficits, personality change, etc). HSV encephalitis can be a devastating condition.
Fungal
Fungal infections usually occur in the setting of HIV or signifiant immunosuppression.
- Cryptococcus neoformans
- Coccidioides immitis
- Candida species
- Histoplasma capsulatum
- Blastomyces dermatitidis
Cryptococcus neoformans is a particularly devastating meningitis that is usually seen in patients with poorly controlled HIV. It is a yeast-like fungus that is a major cause of HIV-associated mortality worldwide.
TB
Mycobacterium tuberculosis (TB) should always be considered in patients who are immunosuppressed. In addition, have a low threshold for suspecting TB meningitis in patients with aseptic meningitis (initial bacterial cultures negative), chronic meningitis (> 7 days duration), or from high prevalence area.
TB affecting the central nervous system may present as an acute meningitis or with more insidious onset. Without prompt treatment, TB meningitis is associated with significant morbidity and mortality.
Neisseria meningitidis
N. meningitidis can cause a rapidly progressive, and devastating meningitis with profound bacteraemia.
N. meningitidis is a gram negative diplococci. It is found as a commensal organism in the upper respiratory tract of 5-11% of the adult population. Around 25% of adolescents are asymptomatic carriers.
The organism typically causes meningitis in patients of all ages, except the very young (< 3 months). The incubation period for infection is usually 2-7 days and the spectrum of disease is wide. Some patients may develop a mild self-limiting illness, whereas others may present with fulminant meningococcal disease characterised by septic shock and multi-organ failure.
The majority of cases are caused by 6 serogroups, which are categorised according to its polysaccharide capsule. These include A, B, C, W, X and Y. More than 95% of cases of meningitis are caused by serogroups B, C and Y.
The UK currently offers N. meningitidis vaccinations to children and young adults as part of the UK vaccination programme. This includes the MenB and MenACWY vaccines.
Streptococcus pneumoniae
Streptococcus pneumoniae is commonly implicated in cases of meningitis, pneumonia, and ear infections.
S. pneumoniae is a gram positive diplococci. It is a very common cause of pneumonia and usually transmitted by close contacts through droplet or direct contact with secretions from the respiratory tract. It colonises 5-10% of asymptomatic adults and up to 40% of children.
Patients at all ages, except the very young (<3 months), are at risk of invasive S. pneumoniae. Similar to N. meningitidis, there are multiple serogroups of the organism based on the constituents of its polysaccharide capsule. This includes over 90 different types but only 8-10 cause serious invasive infections.
The UK currently offers S. pneumoniae vaccination to children as part of the UK vaccination programme. It also offers the vaccine to patients with significant co-morbidities (e.g. diabetes mellitus) and all adults > 65 years old.
Pathophysiology
Bacterial meningitis is usually transmitted through droplets or secretions from the upper respiratory tract.
Here, we predominantly discuss the pathophysiology of bacterial meningitis.
Colonisation
Bacterial meningitis is usually acquired through droplets or secretions from the upper respiratory tract. This is because bacterial pathogens implicated in meningitis usually colonise the upper respiratory mucosa. Other common sites of colonisation include the lower respiratory airways, gastrointestinal tract and lower genital tract.
Routes of infection
Bacteria cause meningeal inflammation by two major routes:
- Invasion of bloodstream: subsequent haematogenous spread to meninges (commonly seen with N. meningitidis and S. pneumoniae)
- Direct contiguous spread: usually as a result of ear, nose or throat infections (e.g. sinusitis, otitis media). Alternatively trauma.
Natural history
Once bacteria penetrate the blood-brain barrier (BBB), they spread quickly within the meninges and can eventually damage underlying brain tissue.
- Mild cases: infection usually confined to the subarachnoid space.
- Severe cases: brain parenchyma underlying pia mater can be affected leading to widespread destruction.
As the immune system is activated, there is infiltration of immune cells and release of inflammatory cytokines. The combination of inflammatory cell infiltration, cytokine-mediated damage and replicating bacteria can perpetuate the infectious process. This leads to a number of complications:
- Damage to cranial nerves
- Obstructive hydrocephalus (disruption of CSF flow, leading to fluid accumulation)
- Local ischaemia (local inflammatory reactions causes vessel inflammation and thrombophlebitis)
- Cerebral oedema: secondary to interstitial oedema (due to altered CSF and venous flow), cytotoxic oedema (due to inflammation reaction) and vasogenic oedema (due to increased BBB permeability)
If left untreated, progressive cerebral oedema leads to diffuse neuronal injury. This is usually combined with systemic complications due to bacteraemia, which include septic shock and multi-organ failure.
Clinical features
Meningitis is characterised by meningism, which describes a triad of headache, neck stiffness and photophobia.
Bacterial meningitis is more likely to present with a sudden onset illness (up to 50% within 24 hours of symptom onset) and systemic upset (e.g. signs of sepsis). Viral meningitis has a slightly longer prodrome and usually less severe symptoms. Meningism is characteristic of both, which describes a triad of headche, neck stiffness and photophobia.
Over 95% of cases of meningitis present with at least two of the following symptoms: headache, fever, neck stiffness, and altered mental status.
Symptoms
- Neck stiffness
- Photophobia: dislike for bright lights
- Headache (>80%)
- Fever (>70%)
- Nausea & vomiting
- Fatigue
- Confusion
- Irritable or unsettled behaviours: particularly in children
- Altered mental status (>70%)
Signs
- Tachycardia
- Hypotension
- Marked neck stiffness
- Photophobia
- Non-blanching rash: concerning sign of meningococcal septicaemia from ‘leaky’ blood vessels. May be seen with other organisms.
- Seizures
- Focal neurological deficits
- Reduced consciousness and coma
Classic signs
- Kernig's sign: inability to fully extend at the knee when the hip is flexed at 90º due to pain
- Brudzinski's sign: spontaneous flexion of the knees and hips on active flexion of the neck due to pain
Both Kernig’s and Brudzinski’s are classical signs of meningeal irritation. However, absence of these signs does not exclude meningitis.
Diagnosis
The diagnosis of meningitis can be challenging.
Due to the potentially life-threatening nature of meningitis (esp. bacterial meningitis), a diagnosis is initially made on clinical suspicion and treatment instigated whilst further investigations are organised.
Definitive diagnosis of an infectious meningitis (e.g. bacterial meningitis) is by isolation of a pathogen from a CSF sample following a lumbar puncture (LP). However, it may be difficult to isolate an organism, especially with administration of antibiotics prior to LP. Given this, a confident diagnosis of bacterial meningitis may be made in patients with a bacterial pathogen isolated from blood cultures with a CSF sample suggesive of infection (e.g. raised WCC), even if cultures are negative.
Initial assessment
Meningitis can be a life-threatening condition, therefore prompt initial assessment is needed to allow initiation of treatment.
Bacterial meningitis should be the foremost consideration in a patient presenting with acute meningism and fever. It is a medical emergency and requires urgent treatment with broad-spectrum antibiotics in line with local antibiotic guidelines. Delays in initiation of antibiotics can lead to a significant increase in morbidity and mortality.
Once bacterial meningitis has been excluded, an alternative diagnosis or another cause of meningitis (e.g. viral) can be considered.
Clinical assessment
Prompt clinical assessment should focus on:
- ABCDE assessment: if critically unwell
- Focused history: important to determine any preceeding illnesses that increase risk of meingitis (e.g. sinusitis, otitis media, contact with an affected patient)
- Allergy status
- Formal examination: assess for meningeal irritation, look for non-blanching rash and signs of sepsis (e.g. hypotension, tachycardia).
A non-blanching rash refers to identification of petechiae (<2mm red lesions) or purpura (>2mm), which are both a sign of abnormal vessel integrity. In meningitis, it is indicative of septicaemia and is most commonly seen in meningococcal infection. Importantly, the disease may already be quite advanced before the rash develops.
NOTE: in the early stages of meningitis and septicaemia, the rash may be blanching. Important to regularly reassess.
Community antibiotic administration
Patients with a suspected meningitis and a non-blanching rash should be given prompt parenteral (intravenous or intramuscular) antibiotics and referred urgently to hospital. Unless there is a clear penicillin allergy, the choice is benzylpenicillin. Paramedics can administer benzylpenicillin for suspected meningococcal meningitis.
Benzylpenicillin dosing for suspected meningococcal meningitis prior to transfer to hospital:
- Children 1-11 months: 300 mg
- Children 1-9 years: 600 mg
- Child 10-17 years: 1200 mg
- Adults: 1200 mg
Investigations
Patients with suspected meningitis undergo a series on investigations including blood tests, cultures, neuroimaging (if indicated) and LP.
Once admitted to hospital, patients are promptly assessed as discussed in the chapter on initial assessment. Durinng assessment, they undergo a series of investigations including blood tests, cultures, neuroimaging (if indicated) and LP.
If there is concern about a non-blanching rash, sepsis, or rapidly-progressive disease, patients should be given urgent intravenous antibiotics (e.g. ceftriaxone 2 g STAT or chloramphenicol if severe penicillin allergy) whilst awaiting further tests.
Bedside tests
- Throat swab
- Respiratory viral screen (nasopharyngeal swab)
Blood tests
- Full blood count
- Urea & electrolytes
- Liver function tests
- Bone profile
- Coagulation
- C-reactive protein
- Blood cultures
- Venous blood gas
- Meningococcal PCR
Imaging
A CT head is often completed as part of the work-up for meningitis. This is predominantly to look for any contraindications to lumbar puncture instead of being used as a diagnostic tool.
Not all patients require a CT head. In general, the main indications include:
- Immunocompromised patient
- History of CNS disease (e.g. tumour, stroke, abscess)
- New-onset seizures (within a week of illness)
- Swollen optic discs: assess with direct ophthalmoscope or slit-lamp
- Abnormal conscious level
- Focal neurological deficit (e.g. limb weakness, cranial nerve palsy)
The reason to perform a CT head in these situations is because of the small, but increased, risk of brain herniation with lumbar puncture with raised intracranial pressure (ICP). On CT, features of raised ICP include effacement of the ventricles, basal cisterns and/or other CSF areas, brain herniation or loss of grey-white matter differentiation. Effacement refers to obliteration of a cavity or space by mass effect (e.g. raised ICP or tumour).
In addition, neuroimaging is useful at looking for an alternative diagnosis or complication of meningitis (e.g. abscess). These would be suggested by the clinical situations described above.
Lumbar puncture
Lumbar puncture with analysis of the CSF is the key investigation in the work-up of meningitis.
Every patient with suspected meningitis should undergo LP and CSF evaluation. Ideally, an LP should be performed before the administration of antibiotics to improve the diagnostic yield of CSF culture. However, in ‘real-life’ clinical practice this is often difficult and therefore antibiotics have usually already been administered.
An LP involves the insertion of a spinal needle into the subarachnoid space and taking a sample of CSF. It is important to determine the opening pressure with the patient lying on their side in a lateral position. This is a surrogate marker of ICP.
CSF samples should be sent to biochemistry and microbiology for the following tests:
- Cell count and differential
- Protein
- Glucose (paired with serum glucose)
- Microscopy, cultures & sensitivity (MC&S)
- Viral PCR
- Save sample (can subsequently be used to run other tests)
- Others (if indicated): cryptococcal antigen (paired with serum), TB PCR
Interpretation
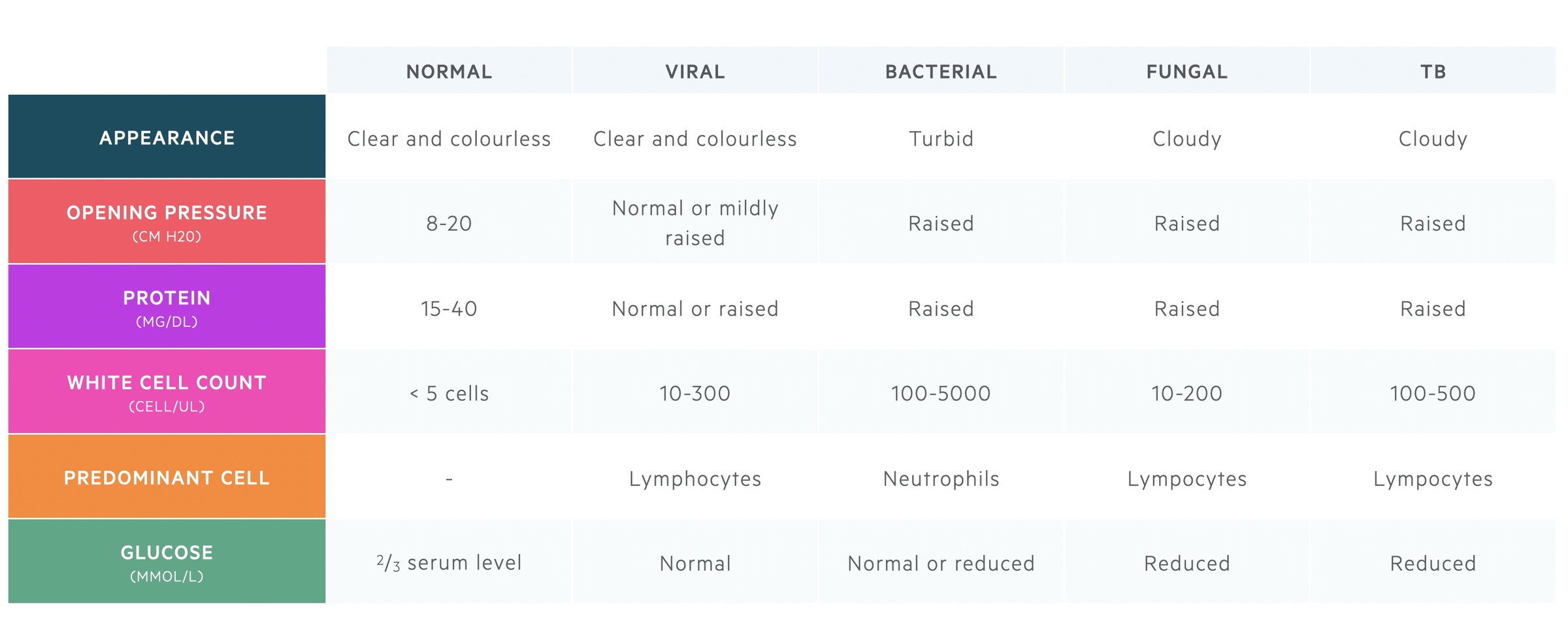
Isolation of a pathogen on MC&S (e.g. bacteria) or PCR (e.g. virus) will confirm the diagnosis. However, this may take time to come back, especially if the initial gram stain for a bacterial pathogen is negative. Therefore, the general appearance, opening pressure, cell count, protein and glucose concentration are used in combination to determine the suspected diagnosis.
Management
In bacterial meningitis, the prompt administration of antibiotics is the principle treatment.
The management of meningitis depends on the underlying aetiology. Often, patients are treated initially for bacterial meningitis due to the potentially devastating consequences left untreated. Once this has been excluded, treatment can be stopped and other diagnoses and appropriate treatment instigated.
Bacterial
The first-line antibiotic for bacterial meningitis is ceftriaxone, which is a 3rd generation cephalosporin. This should be given as 2 grams twice daily. It should be used with caution in patients with penicillin allergy due to cross-reactivity. If there is a severe penicillin allergy (e.g. anaphylaxis) it should be avoided. Further choice antibiotic choices depends on the suspected, or isolated, bacterial pathogen (e.g. amoxicllin if concern about L. monocytogenes.
The second line antibiotic is chloramphenicol (~25 mg/kg, max dose 1 gram QDS). This should be given to those with a severe penicillin allergy. In these situation it is usually recommended to discuss with microbiology.
Dexamethasone can be considered as a treatment adjunct in patients highly suspected of having bacterial meningitis, especially pneumococcal meningitis. It may reduce development of neurological complications. It should be given in close association with the initial antibiotic dose for maximal effect. It can be continued in confirmed S. Pneumoniae meningitis.
Viral
The majority of cases of viral meningitis are secondary to enteroviruses and do not require any specific treatment.
Supportive treatment with rest, hydration, analgesia and antipyretic can be considered. If there is concern about encephalitis or HSV infection then aciclovir can be considered.
NOTE: treatment of fungal and TB meningitis are beyond the scope of these notes.
Public Health England (PHE)
Acute meningitis is a notifiable disease.
Meningitis is a notifiable disease and many pathogens that cause meningitis (e.g. N. meningitidis, invasive S. pneumoniae) are notifiable organisms. This means PHE, usually via notification of the ‘proper officer’ at a local council or local health protection unit, need to be informed once the disease is confirmed or organism isolated.
Prevention
Contact tracing and chemoprophylaxis should be considered in close contacts following a case of acute meningitis.
Contact-tracing
This involves identifying individuals who have come into close contact with the patient affected by meningitis. This is usually close household contacts. Contact-tracing is orchestrated by the local health protection team.
Examples of close contacts:
- Prolonged contact within 7 days of illness onset (e.g. household member, sharing halls of residence, partner)
- Transient close contact but exposed to large respiratory secretions (usually around time of hospital admission)
Chemoprophylaxis
This is the administration of antibiotic prophylaxis in at-risk close contacts to prevent the risk of developing acute meningitis in case they have asymptomatic carriage of the pathogen
This should be completed following confirmed cases of meningococcal meningitis regardless of vaccination status. Antibiotic choices in adults:
- Ciprofloxacin: 500 mg single dose
- Rifampicin: 600 mg twice daily for two days
Vaccination
The UK vaccination programme is important for prevention of bacterial meningitis. The programme includes vaccines against H. influenza, N. meningitidis, S. pneumoniae.
- Hib vaccine: protects against H. influenzae type B. Given as part of childhood vaccination programme, in patients with complement deficiency and hyposplenism
- MenB and MenACWY vaccines: protects against different serogroups of N. meningitidis. Given as part of childhood vaccination programme, in patients with complement deficiency and hyposplenism
- Pneumococcal conjugate vaccine: protects against 13 serogroups of S. pneumoniae. Given as part of childhood vaccination programme, to all adults > 65 years old and those with major co-morbidities (including complement deficiency and hyposplenism).
Vaccination against encapsulated bacteria is important in patients with hyposplenism. This is because encapsulated bacteria are poorly opsonised (i.e. attachment of complement) and reliant on removal by resident splenic phagocytes. Without a spleen, patients are at risk of overwhelming infections.
Complications
Without prompt recognition and treatment, bacterial meningitis is a life-threatening condition that can lead to death.
Immediate complications
- Severe sepsis and multi-organ failure
- Seizures (20-30% of adults)
- Cerebral oedema
- Death
Chronic complications
If patients recover, a number of long-term complications may develop
- Hearing loss and deafness
- Seizure disorder
- Focal paralysis
- Cranial nerve defects
- Hydrocephalus
- Cognitive impairment
- Gait disturbance
- Peripheral gangrene
- Blindness
Waterhouse–Friderichsen syndrome
This describes a classical syndrome causing primary adrenal insufficiency (Addison's disease) due to bilateral adrenal gland haemorrhage. It usually occurs in association with severe bacterial infection such as meningococcal septicaemia.
Last updated: May 2022
Have comments about these notes? Leave us feedback
