Neonatal jaundice
Notes
Overview
Jaundice is one of the most common medical conditions affecting neonates.
Jaundice refers to the yellow discolouration of skin and mucous membranes that occurs due to an increase in the level of bilirubin. Bilirubin is one of the main breakdown products of haemoglobin, which is the oxygen carrying molecule in our red blood cells.
Neonatal jaundice refers to jaundice that occurs in preterm or term babies within the first month of life (the neonatal period). It may be a normal physiological response or due to a pathological disorder with serious consequences.
Due to the potential short and long-term consequences of neonatal jaundice, careful assessment as to the cause, severity and trajectory of neonatal jaundice is required in each case.
Epidemiology
Neonatal jaundice is extremely common affecting approximately 60% of neonates at term.
Neonatal jaundice is one of the most common medical conditions to affect newborns. It is more common in preterm babies.
- Term ( ≥ 37 weeks gestation): affects 60% of newborns.
- Preterm (< 37 weeks gestations): affects 80% of newborns
Aetiology
Neonatal jaundice is most commonly due to ‘physiological’ jaundice, which occurs within the first weeks of life.
There are multiple causes of neonatal jaundice. The majority of cases occur secondary to physiological jaundice within the first weeks of life. Pathological jaundice refers to a wide range of causes, some more severe than others. It may coexist with physiological jaundice. If neonatal jaundice extends beyond 14 days of life, we call it prolonged jaundice.
Physiological jaundice
This refers to the generally harmless jaundice, which occurs within the first few weeks of life. There is no underlying cause, although it is more common in newborns who are breastfed for unknown reasons. Most cases appear at 2 days of age and subsequently decreases by day 10.
Key factors that predispose to physiological jaundice:
- Red blood cells: shorter lifespan in neonates. Due to higher turnover, bilirubin levels greater.
- Metabolism, circulation and excretion of bilirubin: slower in neonates
Breastfeeding is closely linked with physiological jaundice. Newborns breastfed are more likely to develop physiological jaundice than bottle-fed. Factors associated with breastfed jaundice include decreased caloric intake, increased enterohepatic cycling and actual constituents of breastmilk.
‘Breastmilk jaundice’ is typically used to refer to a prolonged physiological jaundice in breastfed newborns.
Pathological jaundice
Jaundice due to an underlying disorder is termed pathological jaundice. There are a number of different causes:
- Haemolysis: blood group incompatibility (ABO, rhesus), glucose-6-phosphate deficiency (G6PD)
- Inborn errors of metabolism: Gilbert syndrome, Crigler–Najjar syndrome
- Obstruction (biliary): congenital obstruction and malformations (e.g. biliary atresia)
- Systemic disease: sepsis, bruising
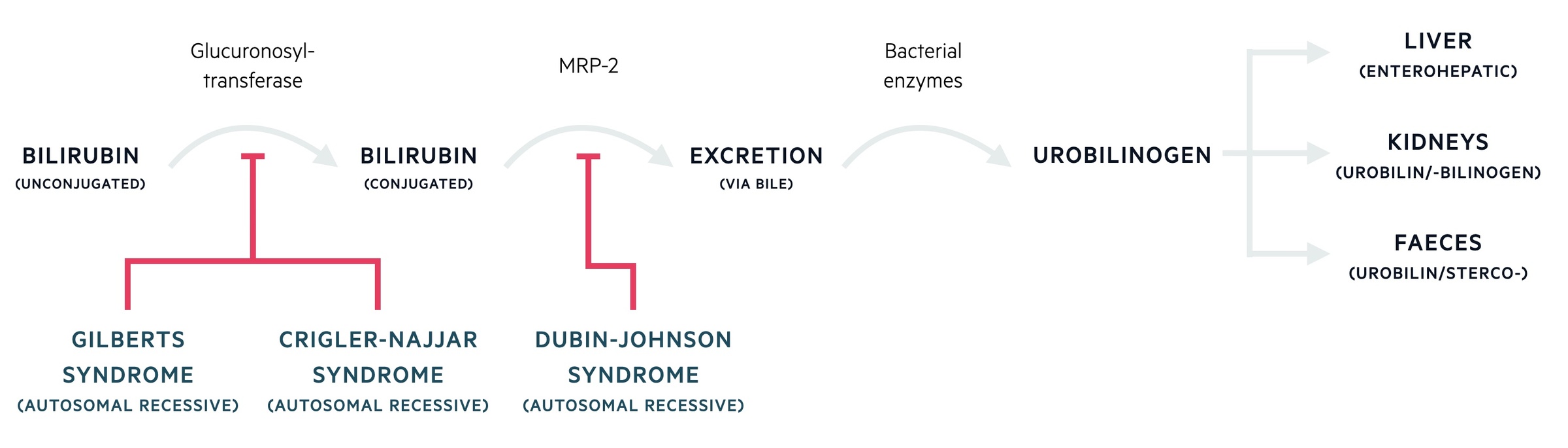
Inborn errors of metabolism
Gilbert and Crigler-Najjar syndrome are disorders of biliary conjugation, which is an important step in bilirubin metabolism that takes place in the liver. They are both due to mutations within the UGT1A1 gene locus, which encodes glucuronosyltransferase. They cause an unconjugated hyperbilirubinaemia. Crigler-Najjar is more severe as it leads to a profound reduction or absence of the enzyme activity, whereas Gilbert’s has mildly reduced activity leading to a benign course.
Risk factors
Preterm babies are more likely to develop neonatal jaundice (< 37 weeks gestation).
Risk factors for the develop of significant neonatal jaundice include:
- Ethnicity: Asian, European, or native American
- Newborn factors: visible jaundice within 24 hours of life, gestational age < 38 weeks, male gender, visible bruising (e.g. cephalhaematoma)
- Maternal factors: diabetes mellitus, >25 years old, exclusive breastfeeding
- Other: previous sibling needing phototherapy for neonatal jaundice
Pathophysiology
Bilirubin is the breakdown product of haemoglobin, which is transferred to and processed by the liver.
Haemoglobin is initially broken down to biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. This unconjugated bilirubin (or indirect bilirubin) is transported to the liver bound to albumin and undergoes conjugation.
Conjugation
This involves processing unconjugated bilirubin in the liver into conjugated bilirubin (or direct bilirubin). It makes bilirubin more water soluble and thus allows excretion through the biliary system via bile. A small proportion is absorbed and excreted by the kidneys.
Conjugation involves a number of steps:
- Addition of glucuronic acid: process known as glucuronidation. Mediated by enzyme glucuronosyltransferase.
- Biliary excretion: active excretion of bilirubin into bile.
- Intestinal modification: further metabolism by bacteria. Degradation to urobilinogen and subsequent excretion in faeces.
- Renal excretion: small proportion enters enterohepatic circulation and reabsorbed. Subsequently excreted by kidneys.
Kernicterus
Unconjugated bilirubin is potentially toxic to neural tissue in newborns and able to cross the blood-brain barrier. Therefore, if hyperbilirubinaemia is left untreated it can lead to devastating short and long-term complications.
Kernicterus is a pathological term, which describes the yellow staining of cerebral tissue, particularly in the deep grey matter of the brain due to bilirubin deposition. In clinically practice, kernicterus is often used to describe the presence of acute or chronic bilirubin encephalopathy (see clinical features). Thankfully, kernicterus is very rare in practice.
Clinical features
Neonatal jaundice presents with yellow discolouration of a skin and mucous membranes.
It can be surprisingly hard to detect jaundice, especially in newborns with dark skin. However, without recognition newborns do not enter the care pathway to prevent serious complications.
Visual/clinical assessment
Assess for risk factors associated with hyperbilirubinaemia. Examine the newborn at every opportunity (within first 72 hours). A proper visual inspection involves checking the newborn in bright, natural light with assessment of sclera, mucous membranes and after blanching skin. If risk factors, a healthcare professional should do a second assessment at 48 hours.
Acute bilirubin encephalopathy
Clinical features associated with deposition of bilirubin in the brain:
- Lethargy
- Irritability
- Abnormal muscle tone / posture
- Apnoea episodes (temporary cessation of breathing)
- Convulsions
Chronic bilirubin encephalopathy
Clinical features associated with prolonged damage to cerebral tissue from untreated hyperbilirubinaemia.
- Cerebral palsy
- Hearing loss (sensorineural)
- Visual (e.g. gaze palsy)
- Dental dysplasia
Diagnosis & investigations
Diagnosis of neonatal jaundice can be made clinically and then confirmed using serum bilirubin measurements.
Confirmation of hyperbilirubinaemia
- Gestational age < 35 weeks or < 24 hours old: serum bilirubin measurement
- Gestational age > 35 weeks and > 24 hours old: transcutaneous bilirubinometer measurement. If level > 250 umol/L proceed to serum bilirubin measurement.
NOTE: if the bilirubin level using transcutaneous bilirubinometer is at or above the relevant treatment threshold for the respective age of the newborn, you should proceed to serum bilirubin measurement.
Monitoring hyperbilirubinaemia
- Under 24 hours old: measure and record bilirubin level within 2 hours. Continue to measure 6 hourly. Stop measurements when bilirubin level below threshold or stable and/or falling. Manage according to threshold graphs
- Over 24 hours old: measure and record bilirubin within 6 hours. Manage according to threshold graphs.
Identifying a cause
A formal clinical examination by a trained healthcare professional should be completed alongside a series of investigations to determine the underlying cause.
Routine screen:
- Serum bilirubin (as above)
- Blood packed cell volume
- Blood group (mother and baby)
- Direct antiglobulin test (DAT): looking for antibody-mediated destruction of red blood cells
If clinically indicated:
- Full blood count
- Blood film
- LFTs
- G6PD levels
- Cultures: work up for sepsis
If prolonged jaundice (> 14 days if term, >21 days if preterm):
- Assess for features of obstructive jaundice (i.e. pale stools, dark urine)
- Conjugated bilirubin levels
- LFTs
- Full blood count
- Urine culture
- Metabolic screen
Management
Management depends on the treatment threshold graph/table and includes photodynamic therapy or exchange transfusion.
Management of neonatal jaundice depends on the extent of hyperbilirubinaema. Treatment is vital to prevent irreversible neurological damage for bilirubin deposition (i.e. kernicterus).
We can used a treatment threshold table for newborns > 38 weeks gestation or treatment threshold graphs for preterm newborns. These provide valuable information about when to instigate management based on the total level of bilirubin.
NOTE: Profound conjugated hyperbilirubinaemia requires specialists input as may suggest severe liver disease (e.g. biliary atresia).
Treatment threshold graph
Each week of gestation has an individual treatment threshold graph. If a baby is born as 30+2 weeks gestation, you would use the 30 weeks gestation graph and day 0 represents the day of birth.
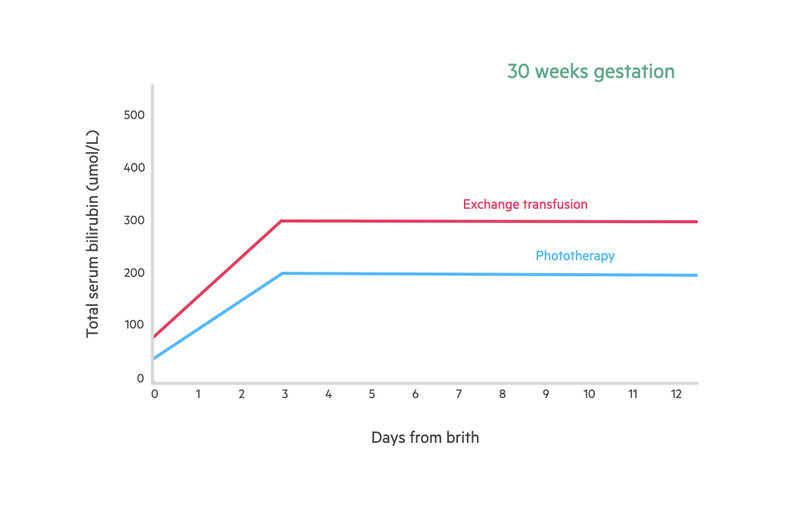
Phototherapy
This involves the use of blue-green light in the range of 460-490 nm, which converts unconjugated bilirubin into water soluble molecules that can be excreted. It is a noninvasive and inexpensive way of treating neonatal jaundice. There are different types including single phototherapy and continuous multiple phototherapy
Newborns having phototherapy require closing monitoring with regular assessment of bilirubin levels (e.g. every 4-6 hours until stable or falling, then interval may be increased).
Red cell exchange
The use of emergency exchange transfusion has decreased with the use of phototherapy. It involves removal of the newborns blood and simultaneously replacing it with a compatible donor. This leads to a dramatic reduction in bilirubin levels.
Last updated: March 2021
Have comments about these notes? Leave us feedback
