Type 1 diabetes
Notes
Overview
Diabetes mellitus refers to a group of metabolic disorders that result from an inability to produce and/or reduced sensitivity to insulin.
Classification
Diabetes mellitus is a chronic, multi-system disease, with profound biochemical and structural sequelae. It can be classified into four main groups:
- Type 1 diabetes mellitus: characterised by an inability to produce/secrete insulin due to autoimmune destruction of the beta-cells (production site of insulin) in the pancreatic islets of Langerhan.
- Type 2 diabetes mellitus: characterised by a combination of peripheral insulin resistance and inadequate secretion of insulin. It is strongly associated with obesity and the metabolic syndrome.
- Gestational diabetes mellitus: new onset of diabetes in pregnancy. It is associated with both maternal and foetal complications and as such patients are managed as part of a multi-disciplinary team in both antenatal and diabetic clinics. Patients with GDM have a higher risk of developing both GDM in future pregnancies and overt diabetes mellitus.
- Other: These can be divided into genetic and acquired disease. Genetic causes refer to monogenic diabetes (i.e caused by mutation to a single gene). They are rare and collectively termed ‘mature-onset diabetes of the young' (MODY). Acquired causes may be secondary to medications or pathological conditions. Common causes include corticosteroids, pancreatitis and pancreatic tumours.
T1DM
Type 1 diabetes is a condition caused by an inability to produce or secrete insulin. It is characterised by an absolute insulin deficiency, state of persistent hyperglycaemia with abnormalities in carbohydrate, fat and protein metabolism.
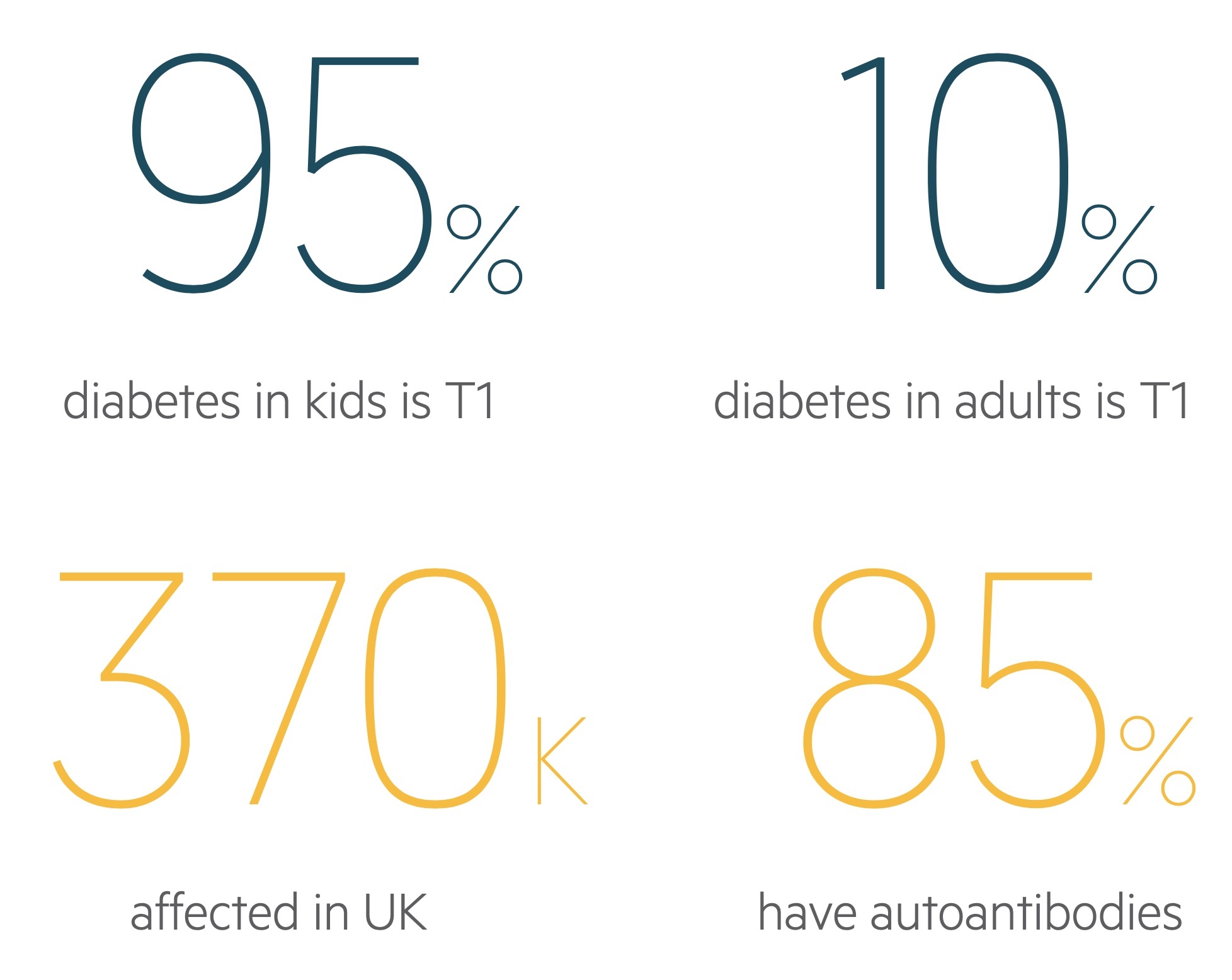
It accounts for 90-95% of diabetes in children, classically presenting with polyuria, polydipsia and weight loss. Insulin is central to management as is monitoring for and treating complications.
Epidemiology
T1DM typically develops in children and adolescents.
The condition can develop at any age. It is estimated that over 370,000 adults are affected with T1DM within the UK and this is thought to represent about 10% of adults who suffer from diabetes. The incidence of T1DM is thought to be increasing.
Aetiology
Insulin is produced and secreted by beta cells within the pancreatic islets of Langerhan.
In T1DM, progressive beta-cell destruction leads to a decline in the amount of insulin that is able to be secreted. This continues until the relative deficiency in insulin is unable to maintain normal blood glucose leading to hyperglycaemia. This usually occurs when up to 90% of the beta-cell mass has been destroyed.
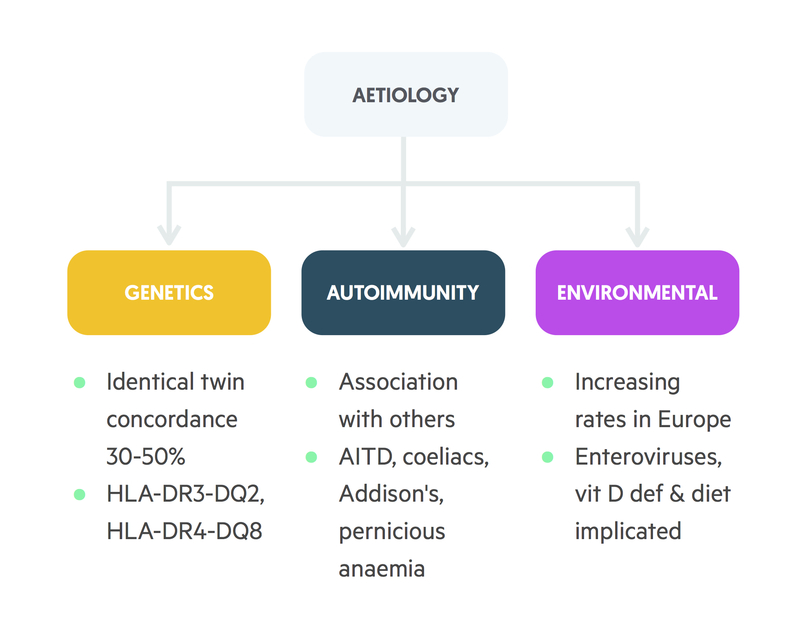
Although the precise mechanism is unknown, it is presumed that autoimmunity is the main factor leading to the destruction of beta-cells. It is thought that genetically susceptible individuals may develop autoantibodies that target the beta-cells in response to an external trigger (e.g. viral infection). Up to 85% of patients with T1DM are found to have circulating autoantibodies. The anti-glutamic acid decarboxylase (anti-GAD) antibody, an enzyme found within beta cells of the pancreas, is most commonly identified.
In T1DM, it is estimated that approximately 15% of patients will have a first-degree relative who has the condition, and there is 30-50% concordance in monozygotic twins. There is also a significant link with other autoimmune conditions. The prevalence of T1DM is higher in patients with autoimmune conditions such as Graves’ disease, autoimmune thyroiditis and Addison’s disease.
T1DM has also been linked to certain human leucocyte antigens (HLA). HLAs are the human form of the major histocompatibility complex (MHC) proteins key to cell-signalling. In particular, they are important for the immune system to be able to distinguish its own cells from pathogens (e.g. from bacteria). The MHC class 1 genes encode HLA-A, HLA-B and HLA-C, which are present on all cells within the body. The MHC class 2 genes encode proteins, which are predominantly found on antigen-presenting cells and immune cells (e.g. T-helper cells). They encode the human leucocyte antigens HLA-DP, HLA-DQ and HLA-DR. It is estimated that up to 95% of patients with T1DM have human leucocyte antigens HLA-DR3 or HLA-DR4.
Physiology
Under normal physiological conditions, glucose metabolism is a tightly controlled process maintaining blood glucose levels between 3.5-8.0 mmol/L.
Insulin, an anabolic hormone that is essential for the synthesis of carbohydrate, fat and protein stores, is the principle hormone that controls glucose metabolism. Numerous hormones and enzymes are involved in glucose metabolism including glucagon and glucagon-like peptide.
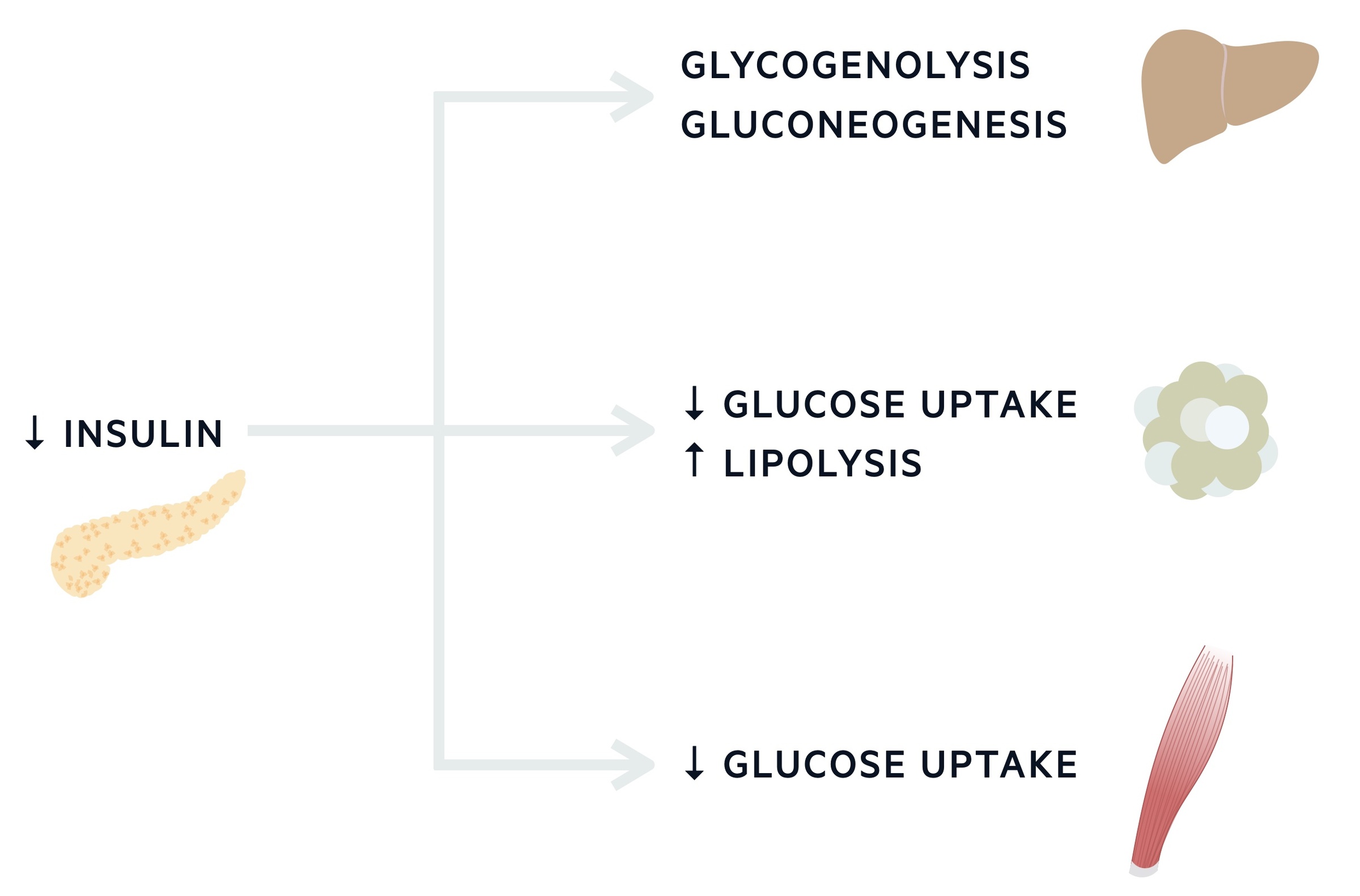
Glucose & insulin during fasting
In normal fasting conditions, insulin concentrations are low as it acts locally on the liver to modulate glucose production. It does this by modulation of glycogenolysis (breakdown of the stored version of glucose termed glycogen) and gluconeogenesis. The latter describes the formation of glucose from non-carbohydrate carbon substrates including amino acids (alanine and glutamate), lactate and glycerol (derived from fatty acids).
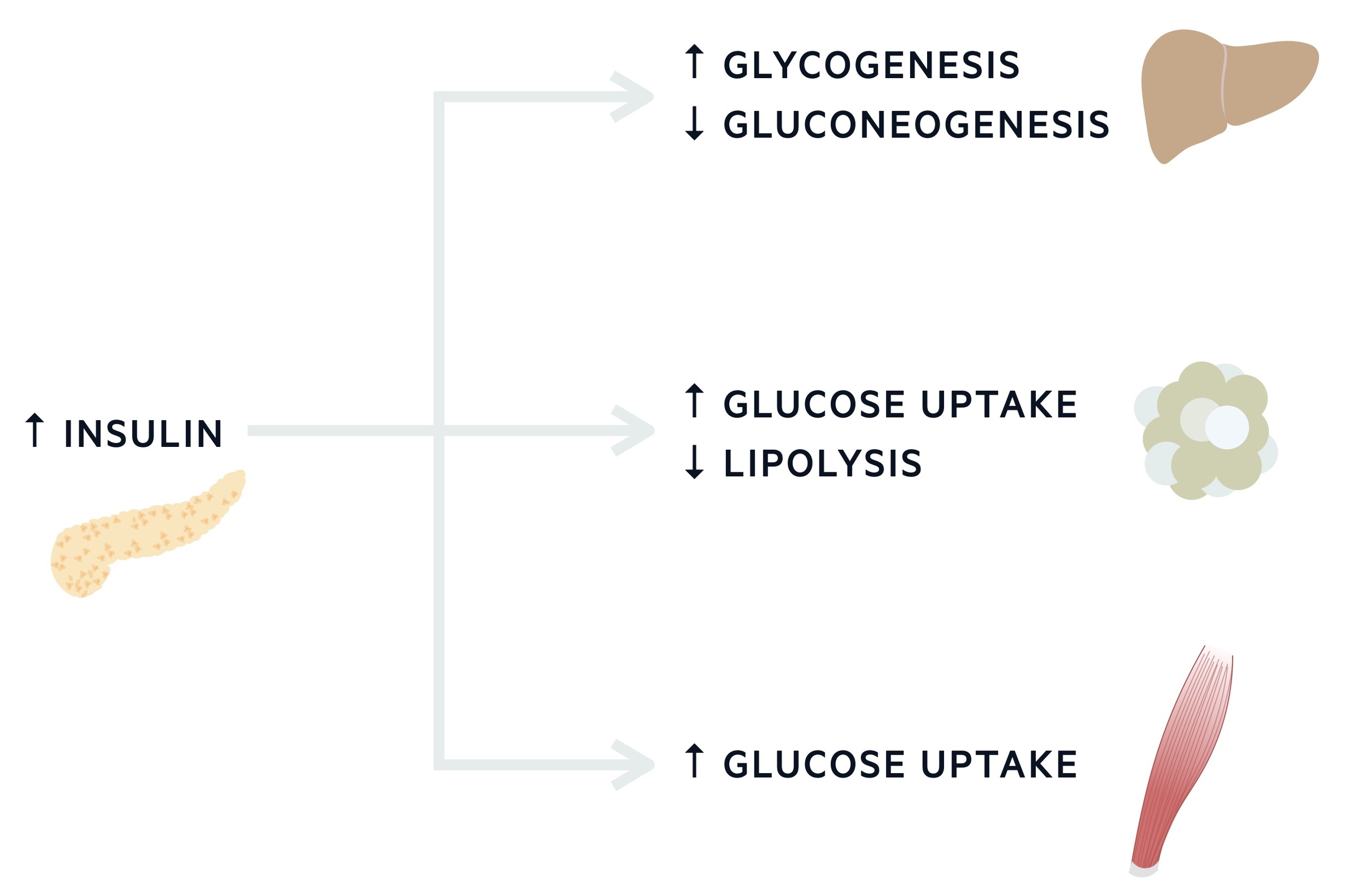
Glucose & insulin post-prandial
Following a meal (post-prandial), insulin is released from the pancreas in large amounts. The release of insulin is enhanced by the release of other gut hormones including glucagon-like peptide (GLP). Insulin acts on the liver to reduce its glucose output, inhibiting glycogenolysis and gluconeogenesis. Insulin is also essential for the promotion of glucose uptake in peripheral tissues (e.g. muscle and adipose tissue).
In addition to this insulin:
- Decreases lipolysis, increases fatty acid and triacylglycerol synthesis.
- Increases glucose uptake in adipose tissue and muscle.
- Increases protein synthesis in a variety of tissues and prevents protein degradation.
Counter-regulatory hormones
Counter-regulatory hormones include glucagon, adrenaline, growth hormone and cortisol. These hormones promote glucose production within the liver (e.g. glycogenolysis, glyconeogenesis) and inhibit peripheral uptake of glucose.
Insulin deficiency
In patients with T1DM, the destruction of beta-cells leads to the progressive reduction in insulin secretion.
Reduced levels of insulin secretion impairs the bodies ability to maintain normal blood glucose levels and hyperglycaemia ensues.
The absence of insulin leads to an increase in the rate of glucose production from the liver and reduced peripheral uptake of glucose. This is exacerbated by the high levels of glucagon and other counter-regulatory hormones. This results in an osmotic diuresis leading to polyuria, polydipsia, dehydration and electrolyte derangement.
The peripheral tissue is unable uptake glucose to utilise it as energy. Weight loss occurs secondary to fluid loss and increased muscle and fat breakdown.
In those with a significant deficiency or additional stresses, a profound catabolic state may occur. The peripheral tissue is unable uptake glucose to utilise it as energy, therefore additional energy sources are required. Fatty acids are taken up into hepatocytes and converted into ketone bodies. Ketone bodies are released back into the circulation and utilised for energy in the form of ATP. The development of ketosis leads to metabolic acidosis (diabetic ketoacidosis). High circulating ketones can induce vomiting, further exacerbating dehydration and electrolyte derangement.
As the process continues the acidosis becomes so severe that pH-dependent enzyme systems start to fail. Left untreated, the condition is invariably fatal with the patient developing acute kidney injury, cerebral oedema and acute respiratory distress syndrome.
LADA
Latent-onset autoimmune diabetes in adults (LADA) refers to a variant of T1DM that occur later in life.
It refers to a group of patients who have autoimmune destruction of beta cells (as evidenced by positive autoantibodies). It tends to have a gradual onset.
It can be suspected in patients who develop diabetes in adult life with associated ketosis, weight loss, low BMI and family history of autoimmune disease. Patients are frequently diagnosed with T2DM whose initial treatments will be ineffectual. Like other cases of T1DM, insulin therapy is required.
Clinical features
The majority of patients will develop T1DM in childhood or adolescence with features of lethargy, polyuria, polydipsia and weight loss.
If these features go unnoticed or progress quickly, patients may present with diabetic ketoacidosis.
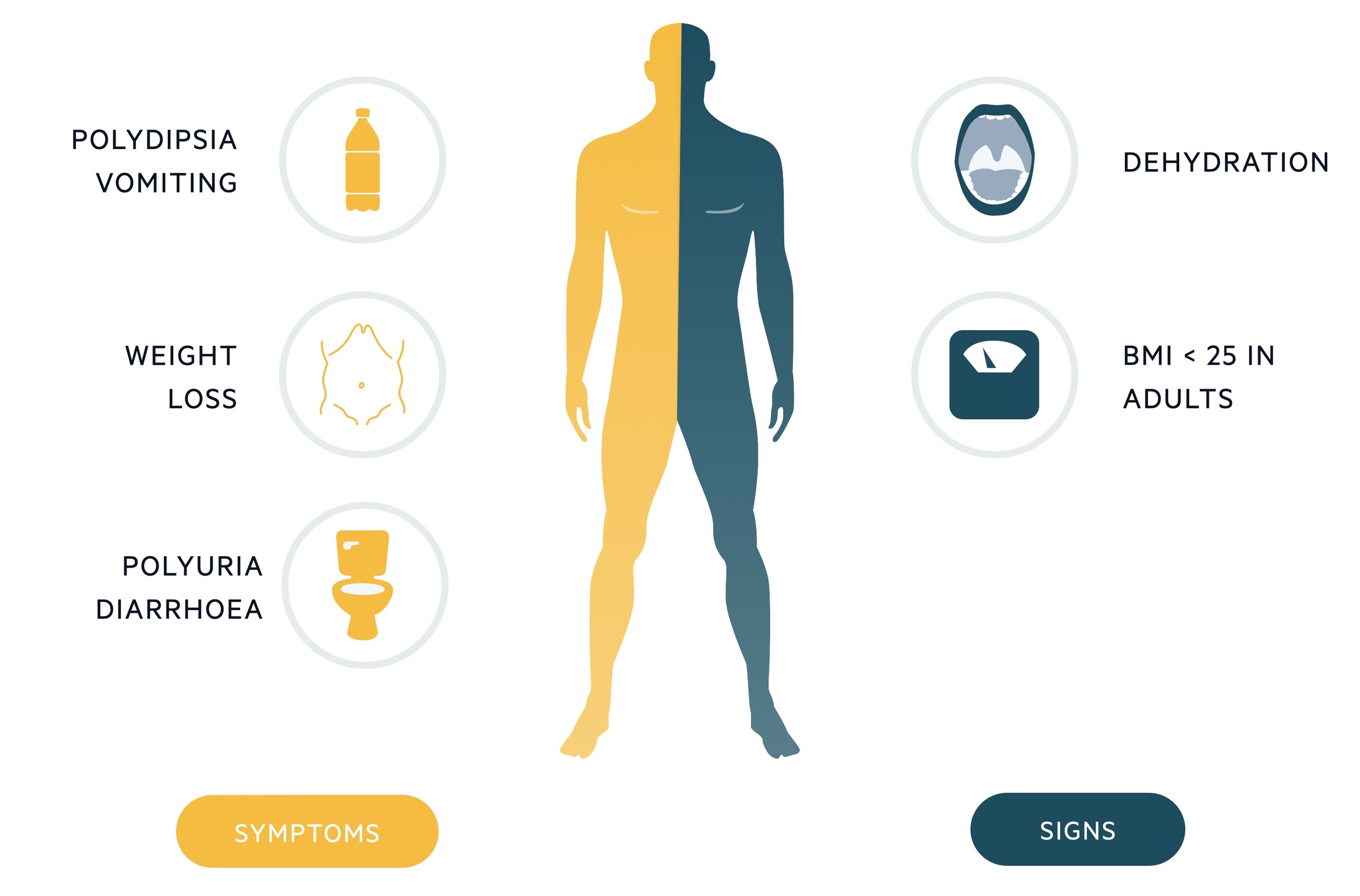
Symptoms
- Polyuria & polydipsia
- Weight loss
- Vomiting
- Lethargy
Signs
- Mild-moderate dehydration (dry skin, dry mucous membranes, reduced skin turgor)
- BMI < 25
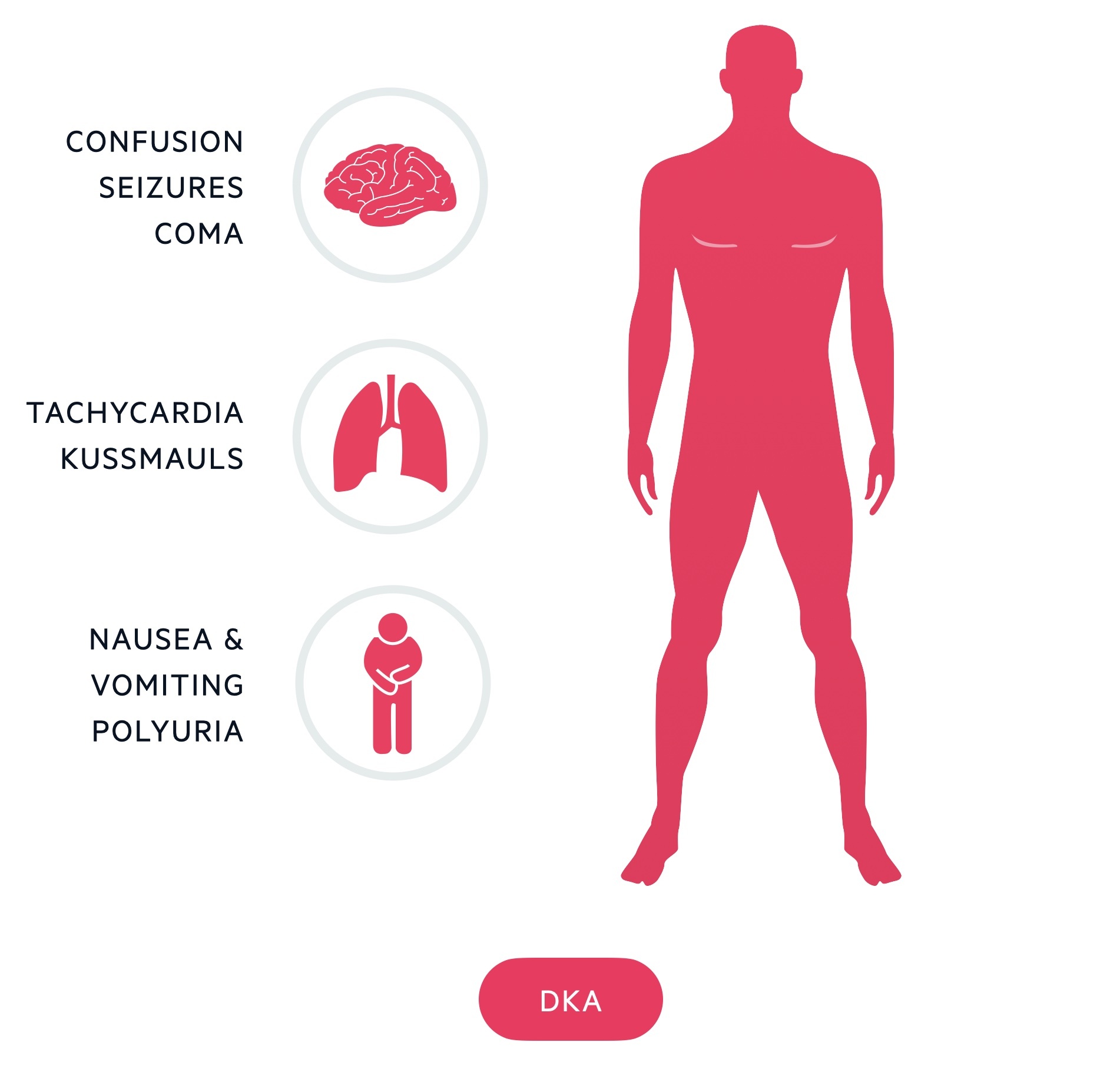
Diabetic ketoacidosis
- Confusion
- Moderate-severe dehydration (sunken eyes, prolonged capillary refill time)
- Vomiting +/- diarrhoea
- Abdominal pain
- Decreased urine output
- Reduced GCS
- Coma
- Shock (tachycardia, hypotension)
- Kussmaul breathing (Deep sighing respiration)
NOTE: Diabetes mellitus is a chronic, multi-system disease that has profound biochemical and structural sequelae. In patients with established chronic disease, it is important to consider clinical features associated with the variety of microvascular and macrovascular complications that can develop. These are discussed further below.
Investigations & diagnosis
T1DM is diagnosed when classical clinical features are found in the presence of a raised random blood glucose level.
The majority of patients with T1DM will be children or adolescents but the diagnosis should not be discounted in adults (LADA).
Patients with suspected T1DM should be referred the same day to a specialist diabetic team for further assessment to confirm the diagnosis and start immediate management. Those presentating with, or suspected of having, a diabetic complication (e.g. diabetic ketoacidosis) need to be investigated and managed accordingly. It is important to consider subtypes of diabetes mellitus at the time of suspected diagnosis with further investigations as necessary (e.g. MODY).
Autoantibodies
Diabetes-specific autoantibodies should be completed in adults presenting with suspected type 1 diabetes mellitus:
- Islet cell antibodies (ICA)
- Glutamic acid decarboxylase (GAD) antibodies
- Insulin antibodies (IAA)
- IA-2 antibodies (target protein tyrosine phosphatase)
It is important to recognise there is a false negative percentage with measuring diabetes-specific autoantibodies. However, the false negative rate is typically lowest at the time or diagnosis and can be improved by testing at least two types. Further testing with serum C-peptide can be considered in patients with negative results and unknown diabetes subtype.
Pancreatic cancer
Patients ≥60 years old presenting with weight loss and new-onset diabetes should be investigated for pancreatic cancer (e.g. CT/MRI imaging of the pancreas).
Management
Management of T1DM requires life-long exogenous insulin to prevent acute complications (e.g. DKA) and long-term sequelae (e.g CKD, IHD, Retinopathy).
This section focuses mainly on the management of adults with T1DM.
Here we will discuss some of the essential aspects to the management of T1DM:
- Insulin use and regimes
- Blood glucose monitoring
- Treatment targets
- Monitoring for complications
- Education
Insulin use and regimes
Exogenous insulin traditionally comes as a parenteral preparation that can be injected subcutaneously. There are numerous types of exogenous insulin, categorised based on the duration of action. These include rapid-acting (e.g. Novorapid), short-acting (e.g. Humulin R), intermediate-acting (e.g. Humulin N), mixed (e.g. Humulin 70/30: Mix of short and intermediate-acting insulin) and long-acting insulin (e.g. Lantus).
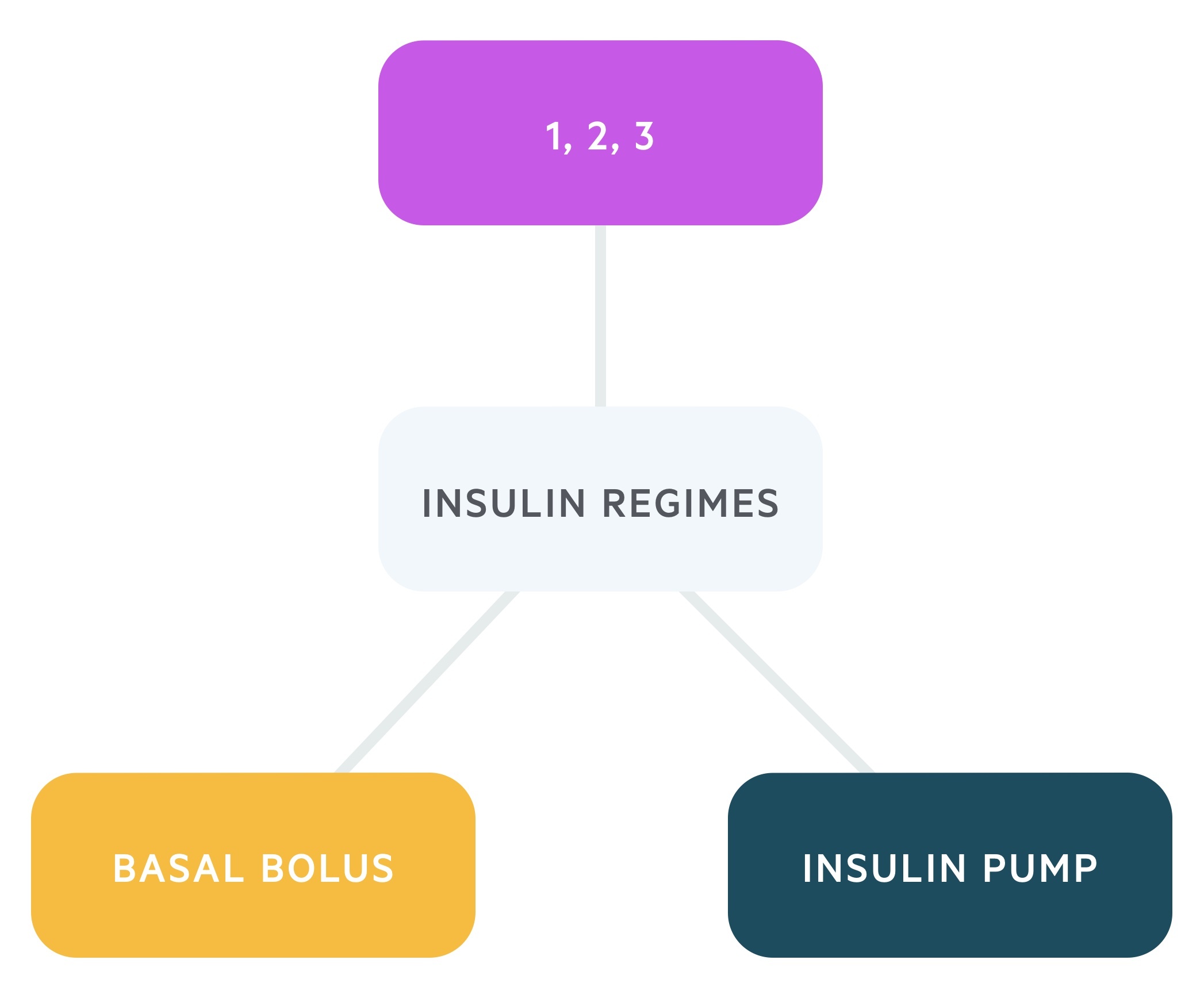
In clinical practice, there are three main insulin regimes that are used in patients with T1DM:
- Basal-bolus regime: typically involves the use of rapid- or short-acting insulin before meals and a long-acting preparation for basal requirements. This regime is thought to best mimic the physiological function of the pancreas in response to meals and provides better flexibility in control of blood glucose. It is the standard approach for patients newly diagnosed with T1DM.
- One, two, or three injections per day regime: traditionally a biphasic regime with the use of both short-acting and intermediate-acting insulin as separate injections or a mixed product.
- Continuous insulin infusion via a pump: supplies rapid- or short-acting insulin. It may be used in patients who are experiencing troubling hypoglycaemic episodes with multiple daily injections regimes.
Blood glucose monitoring
Traditionally, all patients with T1DM were advised to monitor their own blood sugars at least four times a day using a capillary finger prick device (e.g. three times before meals and once before bed). However, patients now have access to a range of modern technologies to add with blood glucose monitoring. All patients with T1DM should be offered continuous blood glucose monitoring known as CGM. There are two types:
- Real-time continuous CGM: latest blood sugars are automatically recorded and shown on a handheld device
- Intermittently scanned CGM (i.e. 'Flash'): a recording is only made when you scan a device over the sensor
Patients' need to be provided with an educational programme on how to use a CGM and this should be continually reviewed as part of their care plan. In patients who do not want to use a CGM, traditional capillary finger prick glucose should be offered. Patients should monitor their blood glucose at least four times a day (before each meal and before bed), but more frequently (i.e. ≥10 times a day) in certain situations (e.g. high-risk job, poor control, frequent hypoglycemia).
Blood glucose targets
The main targets are detailed below:
- On waking: fasting blood glucose 5–7 mmol/L
- Before meals: blood glucose 4–7 mmol/L
- Post meals: test after 90 minutes, blood glucose 5–9 mmol/L
Treatment targets
Long-term control is monitored with HbA1c. This blood test is a measure of glycated haemoglobin, indicative of the average blood glucose over 3 months. It should be repeated every 3-6 months to assess glycemic control.
Patients and clinicians should target a HbA1c < 48 mmol/L (6.5%). Factors that may demand a higher threshold include hypoglycaemic episodes, occupation and co-morbidities.
Monitoring for complications
A regular diabetic assessment looking at all aspects of care, including surveillance for complications, is essential for all patients to improve morbidity and mortality.
On an annual basis (more frequently if required), patients should receive a diabetic review. This includes assessment of injection site problems, retinopathy, nephropathy, diabetic foot problems (e.g. neuropathic problems), cardiovascular risk factors and thyroid disease.
- Retinopathy: annual screening
- Nephropathy: renal function (eGFR) and albumin:creatinine ratio (ACR)
- Diabetic foot problems: full examination including footwear, monofilament assessment of neuropathy, vascular assessment +/- dopplers.
- Cardiovascular risk factors: primary/secondary prevention strategy with optimisation of blood pressure, lipids, weight, smoking and others
- Thyroid disease: screening blood test
- Dental disease (periodonitis): advise regular oral health review
Education
The Dose Adjustment For Normal Eating (DAFNE) programme is aimed at providing type 1 diabetic patients with a way of calculating the amount of carbohydrate in each meal to adjust their insulin accordingly.
The DAFNE mission statement:
“Our vision is to improve outcomes for people with Type 1 diabetes through high quality structured education which is embedded in the Health Service.”
Special circumstances
Patients with diabetes mellitus should feel they have autonomy over their condition, with the ability to seek specialist advice from a diabetic team when needed.
Honeymoon period
The honeymoon period can occur in newly diagnosed patients in whom there is residual beta cell function.
This may negate the need for exogenous insulin for a period. The honeymoon period can last for weeks to months before a deterioration in glycemic control and the need for exogenous insulin.
Sick-day rules
Understanding what to do during intercurrent illnesses is essential in patients with T1DM to prevent poor glycemic control and potential ketoacidosis.
‘Sick-day rules’ refer to a number of recommendations in T1DM with an intercurrent illness. Advice includes:
- Continue insulin therapy, alterations may be required, advice from a specialist may be sought
- Increase frequency of blood glucose monitoring
- Consider ketone monitoring
- Maintain good hydration and when possible a normal meal pattern, meals may be replaced by carbohydrate based drinks
- Seek urgent medical attention if unable to tolerate oral intake, drowsy or sustained vomiting
Acute complications
The two main acute diabetic complications seen in patients with T1DM are hypoglycaemia and diabetic ketoacidosis.
These conditions are associated with a significant morbidity and mortality if not recognised and managed appropriately. Our notes on each topic can be found:
Microvascular complications
Uncontrolled diabetes results in small vessel disease affecting the kidneys, nerves and retinas (amongst other systems).
Retinopathy
Diabetic retinopathy is one of the major eye-related disorders that can occur in patients with diabetes. More than 95% of patients will have detectable changes after 20 years. Other disorders include cataracts and ocular palsies.
Persistent damage to the retina leads to areas of ischaemia and release of angiogenic factors such as vascular endothelial growth factor (VEGF). This promotes new formation of vessels that are weak and friable. This leads to complications including haemorrhage, fibrosis and retinal detachment.
There are a number of classification systems, here we will use the NSC-UK classification.
- Non-proliferative:
- Background (R1): dot and blot haemorrhages, hard exudates, cotton wool spots
- Pre-proliferative (R2): intraretinal microvascular abnormalities (IRMA), venous beading
- Proliferative:
- Proliferative (R3): new vessels at the disc and elsewhere (NVD, NVE), fibrosis, traction retinal detachment
- Maculopathy: exudates, oedema, NVE
Good glycemic control and regular screening to assess early changes are key to preventing and managing disease. Photocoagulation is used to manage proliferative disease. The aim of photocoagulation is to burn holes within the ischaemic retina to prevent the release angiogenesis factors (e.g. VEGF), which stimulate new vessel formation.
Nephropathy
Diabetic nephropathy usually develops 15-25 years following the onset of diabetes and can progress to end-stage renal disease (ESRD).
The earliest sign of diabetic nephropathy is the presence of microalbuminuria, which can be assessed with an albumin:creatinine ratio (ACR). An ACR 3 - 30 mg/mmol is suggestive of microalbuminuria, now categorised as moderately increased albuminuria.
A1: < 3 mg/mmol, normal or mild increase
A2: 3 - 30 mg/mmol, moderately increased
A3: > 30 mg/mmol, severely increased
Microalbuminuria is a marker of systemic microvascular damage and patients should be treated with an ACE inhibitor even in the presence of normotension. Chronic kidney disease (CKD) in diabetes is evidenced by a persistently low eGFR < 60 mmol/L and/or an ACR persistently > 3 mg/mmol/L.
Neuropathy
Diabetic neuropathy refers to a collection of conditions that result from glucose-related damage to neurones of the somatic and autonomic nervous systems.
The main types of diabetic neuropathy are listed below.
- Symmetrical polyneuropathy: typically a peripheral neuropathy that occurs in the leg secondary to loss of vibration, pain and temperature sensation.
- Mononeuropathy: damage to a single cranial or peripheral nerve (e.g. third nerve palsy).
- Diabetic amyotrophy: a spectrum of disease affecting the lumbosacral plexus leading to symmetrical pain, weakness and wasting in the proximal muscles of the leg.
- Autonomic neuropathy: a spectrum of conditions related to damage of the autonomic nervous system, which can effect multiple systems.
Autonomic complications of diabetes can have quite profound effects on patients and may require multi-disciplinary input into their care. Some of the major autonomic neuropathies include postural hypotension, gastroparesis (delayed gastric emptying leading to vomiting), diarrhoea, bladder dysfunction and erectile dysfunction.
Diabetic foot
Diabetic foot problems refer to a wide range of pathologies that are thought to occur secondary to the combination of peripheral neuropathy and poor vascular supply.
Due to loss of sensation and poor blood supply, patients are at risk of a number of complications including diabetic ulcers, secondary infection (e.g. cellulitis, osteomyelitis), skin necrosis and eventually amputation.
Another well-known problem is Charcot’s joint. This is a complex neuropathic arthropathy that results from loss of sensation and subsequent repeated micro-trauma to the foot (traditionally the mid-foot). Microtrauma in the presence of poor peripheral blood flow leads to remodelling, swelling and distortion of the whole joint.
It is estimated that 10-15% of diabetic patients will develop a foot ulcer during their illness and approximately 50% of diabetic hospital admissions are related to diabetic ulcers and foot problems. The management of diabetic foot disease is essential to help recognise and treat complications early. There are now recognised diabetic foot services that are available to help manage patients with these conditions.
Macrovascular complications
Diabetes is a significant risk factor for atherosclerosis and related macrovascular complications.
Optimisation of cardiovascular risk factors on an annual basis is essential to prevent premature cardiovascular disease. This involves:
- Smoking cessation
- Nutritional support & exercise
- Consideration of anti-lipid therapy (e.g. atorvastatin)
- Blood pressure control (e.g. ACE inhibitor)
For further information on the management of macrovascular complications including indications for drug therapy, management targets and referral pathways, please see the NICE guidance on the management of type 1 diabetes mellitus.
Last updated: December 2022
Have comments about these notes? Leave us feedback
