Oesophageal cancer
Notes
Overview
Oesophageal cancer is the 14th most common malignancy in adults in the UK.
The oesophagus is a muscular tube that is situated within the thorax and runs from the pharynx to stomach. It is pivotal in the transfer of food material to the stomach and broadly divided into upper, middle and lower.
There are two major types of cancer that arise from the oesophagus, depending on the cell of origin.
- Squamous cell carcinoma (SCC): usually located in the upper or middle oesophagus. Accounts for >90% of cases worldwide.
- Adenocarcinoma (AC): usually located in the lower oesophagus. Due to chronic reflux and development of a columnar metaplasia, which is a precursor lesion known as Barrett’s oesophagus.
Rarer forms of oesophageal cancers include small cell carcinoma, sarcoma, lymphoma, melanoma and choriocarcinoma.
The hallmark clinical feature of oesophageal cancer is dysphagia, which refers to difficulty swallowing. This is due to obstruction of the oesophageal lumen.
Epidemiology
The incidence of oesophageal adenocarcinoma, particularly at the gastro-oesophageal junction, has increased dramatically.
Oesophageal cancer is rare in young people and more common as we age. The peak incidence is seen in the 7-8th decades.
Globally, SCC is the most common cause of oesophageal cancer, but the incidence of AC is increasing, particularly in Western countries. This is due to an increase in AC risk factors such as obesity.
AC is more commonly seen in men, whereas the male-to-female ratio is more equal in SCC.
Aetiology
Smoking and alcohol consumption are the two major risk factors for the development of SCC.
Oesophageal neoplasia develops due to sequential mutations that occur in the oesophageal epithelium, which allows cells to proliferate uncontrollably. Several risk factors for both SCC and AC increase the likelihood of developing these mutations.
Squamous cell carcinoma
The biggest risk factors for development of SCC include alcohol consumption and smoking.
- Smoking
- Alcohol consumption
- Foods containing N-nitroso compounds
- Chewing of areca nuts
- Previous partial gastrectomy
- Atrophic gastritis
- Human papillomavirus (HPV): mainly genotypes 16 and 18
- Tylosis: rare condition leading to hyperkeratosis of hands and feet
Adenocarcinoma
The majority of AC cases arise from Barrett’s oesophagus, which refers to columnar metaplasia of the lower oesophagus due to chronic reflux. This a pre-malignant lesion.
- Chronic reflux
- Barrett’s oesophagus: 30-fold increase risk of AC
- Smoking
- Obesity
- Zollinger-Ellison syndrome: gastrin-secreting tumour leading to excess hydrochloric acid.
Pathophysiology
The majority of oesophageal SCCs arise from the mid-oesophagus and ACs from near the gastro-oesophageal junction (GOJ).
Squamous cell carcinoma
The majority of SCCs occur due to chronic alcohol consumption and smoking, which damage cellular DNA. This leads to development of genetic mutations that promote abnormal cell growth. Overtime, the cells proliferate uncontrollably leading to invasive cancer.
SCC may be seen as an infiltrating and ulcerated mass in the middle oesophagus, especially if advanced. There is early invasion into surrounding lymph nodes and the tumour may metastasize to liver, bone and lung.
Adenocarcinoma
Typically, chronic reflux leads to inflammation and damage of the lower oesophageal mucosa. This results in columnar metaplasia, which refers to the transformation of the mature squamous cell type to columnar cell type.
This metaplastic epithelium may become dysplastic, which refers to cells with abnormal growth and development. The dysplastic epithelium acquires further genetic mutation that promotes development of invasive carcinoma. AC is most commonly located near the GOJ and there is usually early lymph node involvement.
Clinical features
The hallmark feature of oesophageal cancer is dysphagia, which refers to difficulty swallowing.
Symptoms
- Constitutional symptoms: fevers, anorexia, lethargy, weight loss
- Dysphagia: difficulty swallowing
- Weight loss: due to tumour-related anorexia and poor nutrition from swallowing difficulties
- Bleeding: haematemesis and melaena
- Pain: typically retrosternal pain
- Aspiration: cough, shortness of breath, fever
- Hoarseness: if there is extension to involve the recurrent laryngeal nerve
Signs
- Lymphadenopathy: if local tumour spread
- Cachexia
- Pallor: due to anaemia
- Hepatomegaly: if metastatic spread
Suspected cancer referral
Early recognition and diagnosis is essential to improve mortality.
The NICE (NG12) guideline outlines recommendations for the referral of suspected cancer cases including upper gastrointestinal cancers. Below details the recommended referral for suspected oesophageal cancer.
Urgent (two week wait) referral
This means referring a patient for appropriate investigations (e.g. gastroscopy) for suspected oesophageal cancer within two weeks. It is usually combined with a clinic appointment and CT imaging.
- Dysphagia, OR
- > 55 years with weight loss and one of the following:
- Upper abdominal pain
- Reflux
- Dyspepsia
Non-urgent referral
This means referral for a non-urgent gastroscopy to assess for oesophageal pathology. Usually performed within 6 weeks.
- Haematemesis, OR
- > 55 years with treatment resistant dyspepsia, OR
- > 55 years with upper abdominal pain and anaemia, OR
- Thrombocytosis with one of the following:
- Nausea/vomiting
- Weight loss
- Reflux
- Dyspepsia
- Upper abdominal pain
- Nausea/vomiting with one of the following:
- Weight loss
- Reflux
- Dyspepsia
- Upper abdominal pain
NOTE: Upper and lower gastrointestinal (GI) investigations should also be considered to investigate for GI malignancy (inc. oesophageal cancer) in all postmenopausal female and male patients where IDA has been confirmed unless there is a history of significant overt non-GI blood loss. British Society of Gastroenterology guidelines 2011.
Diagnosis
Oesophageal cancer is diagnosed using upper GI endoscopy and biopsies of suspected lesions.
The principle test for the diagnosis of oesophageal cancer is an upper GI endoscopy known as a gastroscopy. This a camera test that allows direct visualisation of the upper gastrointestinal tract including oesophagus and gastro-oesophageal junction.
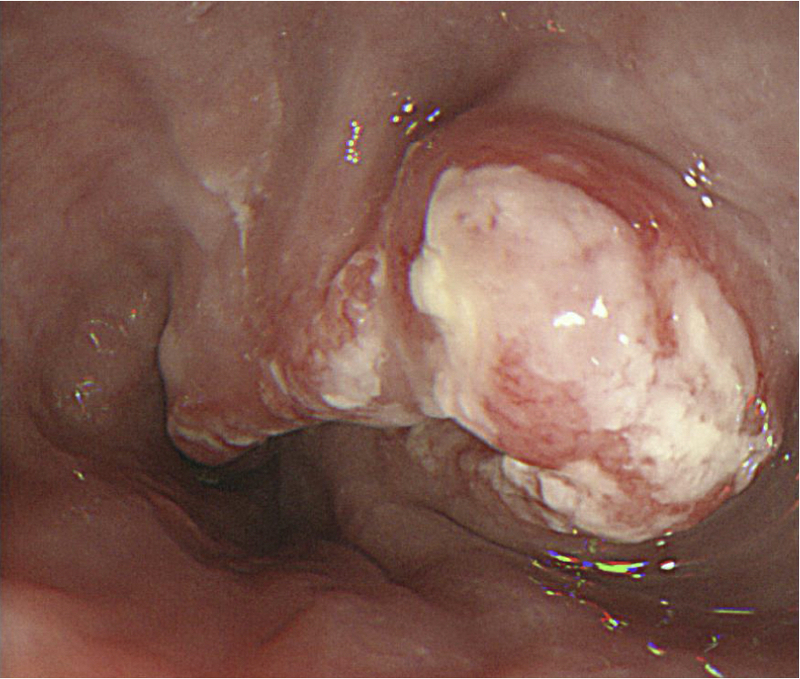
Oesophageal cancer seen at endoscopy
Image courtesy of Wikimedia Commons
Biopsies are taken at the time of endoscopy of any suspicion lesions. These are subsequently sent for histological analysis to see if any features of malignancy are present.
Investigations
Further investigations allow assessment of distant spread and key-organ function to help guide management.
Bloods
- Full blood count
- Serum iron, transferrin saturation, total iron binding capacity (TIBC)
- Urea & electrolytes
- Liver function tests
- Bone profile
- Clotting screen
- Renal function
Imaging
- CT chest/abdomen/pelvis: patients with suspected oesophageal cancer undergo CT imaging to help stage the cancer. See staging below.
- Abdominal ultrasound: may be used to assess for liver metastasis. Usually superseded by CT.
- PET-CT: offered to patients with potentially resectable disease (i.e. candidates for surgery) to assess for distant disease not detected by conventional CT.
Special
- Gastroscopy: principle investigation for diagnosis.
- Endoscopic ultrasound (EUS): can be performed at time of endoscopy. Sometimes completed to help more accurately stage oesophageal cancer if it will change management.
- Diagnostic laparoscopy: may be used to more accurately stage oesophageal cancer if it will alter management.
HER2 testing
Human epidermal growth factor receptor 2 (HER2) testing should be completed on tumour or biopsy specimens. Targeted therapy against the HER2 receptor may be offered to patients with HER2 positive metastatic oesophageal cancer.
Staging
The stage of a cancer describes the extent of cancer spread.
The stage of a cancer is critical to determine treatments, but can be very complex and important mainly for clinicians involved in treating patients with cancer. Stage also provides prognostic information.
Cancer stage is based on tumour size, presence of lymph node involvement and distant spread. We call these three factors ‘TNM’ (tumour, nodes, metastasis).
Tumour
- TX: Primary tumour cannot be assessed
- T0: No evidence of primary tumour
- Tis: Carcinoma in situ/high-grade dysplasia
- T1: Tumour invades lamina propria or submucosa
- T1a: Tumour invades mucosa or lamina propria or muscularis mucosae
- T1b: Tumour invades submucosa
- T2: Tumour invades muscularis propria
- T3: Tumour invades adventitia
- T4: Tumour invades adjacent structures
- T4a: Tumour invades pleura, pericardium, diaphragm or adjacent peritoneum
- T4b: Tumour invades other adjacent structures such as aorta, vertebral body or trachea
Node
- NX: Regional lymph nodes cannot be assessed
- N0: No regional lymph node metastasis
- N1: Metastasis in 1–2 regional lymph nodes
- N2: Metastasis in 3–6 regional lymph nodes
- N3: Metastasis in 7 or more regional lymph nodes
Metastasis
- MX: Distant metastasis cannot be assessed
- M0: No distant metastasis
- M1: Distant metastasis
NOTE: Non-regional lymph node spread is considered M1a disease.
Management principles
The management of oesophageal cancer depends on the extent of cancer and patient fitness.
Treatment options
There are numerous treatment options for the management of oesophageal cancer:
- Surgery: resection of oesophageal or gastro-oesophageal tumours (e.g. oesophagectomy).
- Endoscopic techniques: mucosal resection or mucosal dissection.
- Radiotherapy: use of high energy rays to destroy cancer cells.
- Chemotherapy: use of anti-cancer medications to destroy cancer cells.
- Targeted cancer drugs: monoclonal antibodies against certain receptors (e.g. HER2).
- Palliative care: use of chemotherapy/radiotherapy or stenting to control disease and/or symptoms without aiming to cure.
- Best supportive care: focus primarily on symptoms and quality of life without systemic treatments.
Determining choice
The choice of treatment depends on whether the cancer is limited, locally advanced or advanced/metastatic.
- Limited: refers to small tumours without lymph node involvement or distant spread
- Locally advanced: refers to larger tumours with/without lymph node involvement but without distant spread
- Advanced/metastatic: refers to metastatic disease with spread to distant sites
Limited and locally advanced
The treatment of choice for limited disease is surgical or endoscopic resection.
Surgical resection, radiotherapy, chemotherapy, or a combination, may be used for patients with limited or locally advanced disease.
- Limited disease (Staging: T1-2, N0, M0)
- Locally advanced disease (Staging: T3-4 or N1-2, M0)
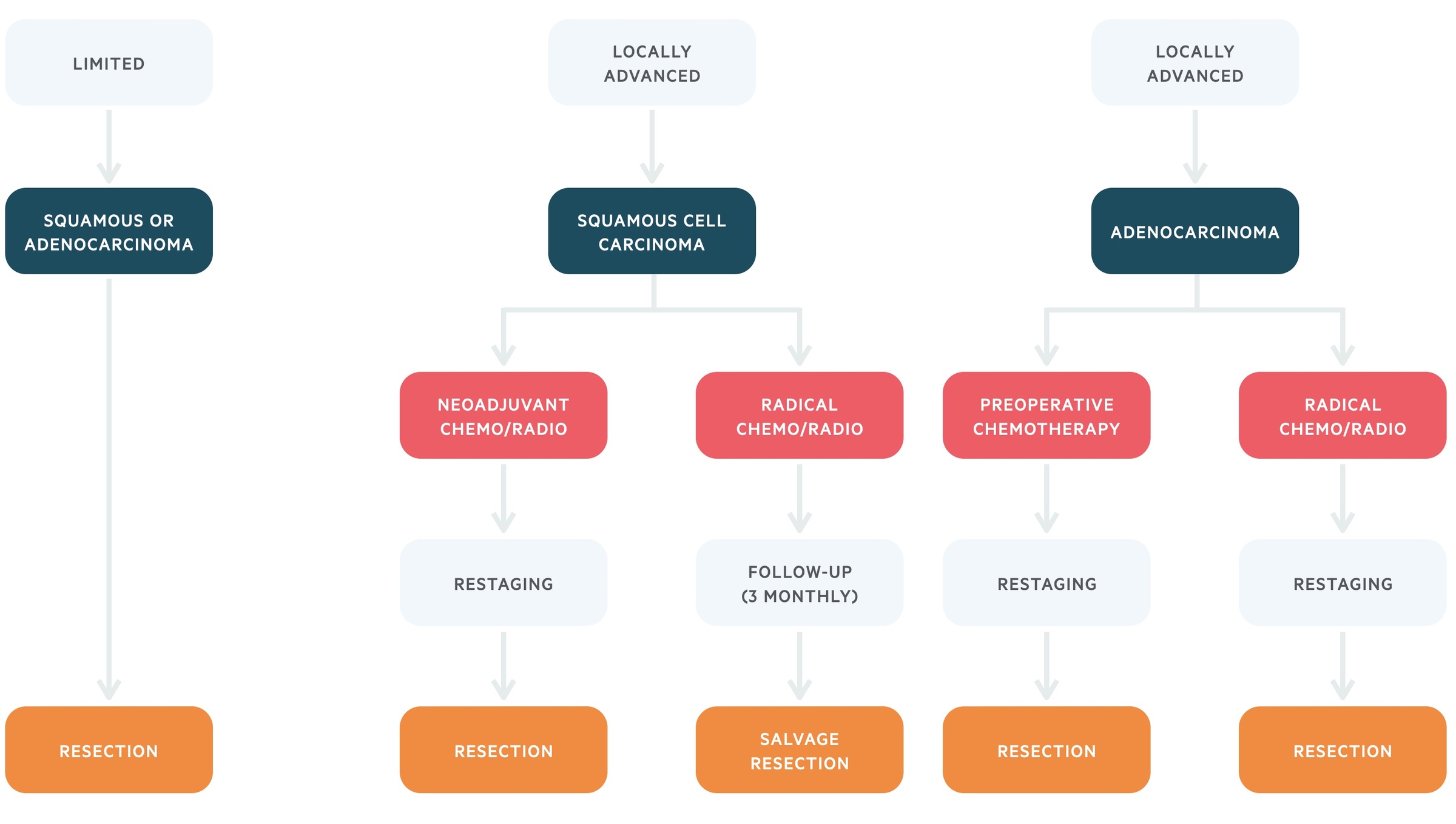
Treatment algorithm based on the European Society of Medical Oncology (ESMO) for limited or locally advanced oesophageal cancer
Surgery
Surgical resection of oesophageal cancer is the treatment of choice if the patient is fit to undergo an operation and the disease is limited. Surgery may be an option for patients with locally advanced oesophageal cancer. This can be considered following systemic anti-cancer therapy (e.g. chemoradiotherapy). The idea is to shrink the tumour, which then becomes resectable.
The surgical technique of choice is an oesophagectomy, which refers to removal of part of the oesophagus and subsequent joining of the remaining oesophagus to the stomach. If the tumour involves the GOJ or proximal stomach then an oesophago-gastrectomy or extended total gastrectomy may be undertaken. In addition to surgical removal of the oesophagus, lymph node dissection may be considered at the time of surgery.
Surgical resection is an option for:
- Limited disease
- Locally advanced disease (deemed resectable following neoadjuvant chemoradiotherapy)
Endoscopic therapy
Patients with limited disease may be suitable for endoscopic therapy to remove the oesophageal cancer.
Two options include:
- Endoscopic mucosal resection (EMR)
- Endoscopic submucosal dissection (ESD)
These are typically performed in specialist upper gastrointestinal centres any may require preoperative assessment with endoscopic ultrasound (EUS) to ensure no localised lymph node spread.
Systemic anti-cancer therapy
The two main systemic anti-cancer therapies used in both SCC and AC are chemotherapy and radiotherapy.
- Squamous cell carcinoma: a combination of chemotherapy (e.g. cisplatin/5-fluorouracil) and radiotherapy may be given as neoadjuvant therapy to shrink the tumour prior to resection or as definite treatment. SCC is highly chemo/radio sensitive, which is why it can be used as radical therapy. Patients undergoing radical chemoradiotheray are followed-up regularly to assess whether a salvage resection should be undertaken.
- Adenocarcinoma: either chemotherapy (e.g. cisplatin/5-fluorouracil) alone or in combination with radiotherapy should be given to patients as neoadjuvant therapy. If there is a response to treatment and the tumour shrinks becoming resectable on restaging then surgical intervention should be considered. Radial chemoradiotherapy (i.e. main treatment to cure cancer) is not an option in AC.
Palliative management
Palliative treatment should be considered in patients with locally advanced disease who are not operative candidates or not fit enough to undergo radical treatment (e.g. poor performance status, extensive co-morbidities).
Options include:
- Radiotherapy: if tumour lies within a radiotherapy field that allows high-doses to be applied.
- Chemotherapy: regimens depend on fitness of the patient.
- Local tumour treatment: endoscopic stenting, palliative radiotherapy.
- Best supportive care: focusing on symptom control only.
NOTE: oesophageal stenting involves endoscopically placing a stent within the oesophagus to keep the lumen open and prevent dysphagia.
Nutritional support
Patients being considered for radical treatment (e.g. surgical resection or chemoradiotherapy) need to have their nutrition optimised. This may mean temporary enteral or parenteral nutrition.
In patients undergoing chemoradiotherapy, they should be considered for enteral nutrition with placement of a radiologically inserted gastrostomy (RIG) tube. This is because chemoradiotherapy will damage the oesophagus leading to localised inflammation during treatment that will impair nutritional intake. A percutaneous endoscopic gastrostomy (PEG) is not suitable in these cases due to the risk of tumour migration from endoscopic pull through of the tube.
Advanced/metastatic
Patients with metastatic disease may be offered a number of palliative treatment options.
Treatment options in patients with advanced oesophageal cancer depends on their symptomatology, fitness (measured by performance status) and personal choice.
Performance status
This describes a patients' level of functioning in terms of their ability to care for themselves, what they can do as part of their daily activities and their physical ability.
Performance status can be measures using the World Health Organisation (WHO) / Eastern Cooperative Oncology Group (ECOG) scale.
- Grade 0: Fully active, able to carry out all normal activities.
- Grade 1: Restricted physical activity but ambulatory and able to carry out light sedentary work (e.g. office work).
- Grade 2: Ambulatory and capable of all self care but unable to carry out any work activities. Out of bed >50% of day.
- Grade 3: Capable of only limited self-care, confined to bed or chair > 50% of waking hours.
- Grade 4: Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair.
- Grade 5: Death.
Palliative treatment
- Radiotherapy: may be used to resolve dysphagia as alternative to stenting.
- Chemotherapy: regimens depend on fitness of the patient.
- Endoscopic stenting: to treat dysphagia.
- Targeted therapy: patients with HER2 positive AC may be offered trastuzumab usually in combination with chemotherapy.
- Best supportive care: focusing on symptom control only.
Prognosis
The five year survival of oesophageal cancer is poor at ~16%
Survival from oesophageal cancer depends on stage. The five year survival for stage 1 disease is 52.8% but only 16% for stage 3 disease. Early diagnosis and treatment is potentially curative, especially if the disease is resectable. However, oesophagectomy is a major operation with significant morbidity and mortality.
Last updated: May 2021
Have comments about these notes? Leave us feedback
