Pulmonary embolism
Notes
Overview
Pulmonary embolism can be a life-threatening condition caused by occlusion within the pulmonary arteries.
Venous thromboembolism
Venous thromboembolism (VTE) is a term that encompasses two conditions:
- Pulmonary embolism (PE): acute/chronic occlusion of pulmonary arteries. Clot breaks off and travels to the lungs (emboli).
- Deep vein thrombosis (DVT): acute/chronic occlusion of deep vein(s). Commonly affects the lower limbs through the formation of a clot (thrombus).
VTE is commonly asymptomatic, but 1-2 per 1000 people every year have symptomatic VTE. DVT accounts for two-thirds of these cases and is commonly seen with PE. It is estimated that 80% of cases of PE are associated with DVT.
Pulmonary embolism
Pulmonary embolism (PE) is defined as occlusion, or obstruction, of the pulmonary artery and/or one of its branches. This may be due to a blood clot, fat, air, or tumour. However, the term is commonly used synonymously with occlusion secondary to an embolus of a blood clot. It is considered a venous thromboembolic (VTE) disease.
Classification
PE can be classified according to the time of onset, clinical severity and location.
Timing
- Acute: presentation at onset of vessel occlusion
- Subacute: presentation within days or weeks of initial event
- Chronic: presentation with complications of chronic emboli (e.g. features of pulmonary hypertension).
Clinical severity
- Non-massive: haemodynamically stable and no evidence of right heart strain
- Sub-massive: haemodynamically stable, but evidence of right heart strain on imaging (e.g. CT, ECHO) or biochemistry (e.g. elevated troponin)
- Massive: haemodynamic instability. Defined as persistently low BP (< 90 mmHg or fall > 40 mmHg) for > 15 minutes or hypotension that requires inotropic support not explained by another cause.
This grading does not refer to the size of the clot. However, a larger clot is more likely to present with haemodynamic instability.
Location
- Segmental and subsegmental: lower order pulmonary vessels. Unilateral or bilateral occlusion
- Lobar: right or left main pulmonary arteries. Unilateral or bilateral occlusion
- Saddle: embolus lodged at the bifurcation of the pulmonary arteries (3-6% of cases).
Aetiology
PE occurs when one or more emboli, usually arising from a thrombus, break off and obstruct within the pulmonary arteries.
VTE occurs as a result of a condition, or state, that increases the risk of blood clots. PE can broadly be divided into provoked (i.e. associated with a condition, or state, that increases the risk of blood clot) or unprovoked (i.e. no identifiable cause for blood clot formation).
- Provoked: transient or persistent risk factors. Typically within three months of event
- Unprovoked: seen in 30-50% of cases. No readily identifiable risk factor for VTE.
The typical cause of PE is an emboli that arises from a DVT. The DVT itself may be provoked or unprovoked based on the same risk factors of PE (it is also a thromboembolic disease). A history of unilateral leg swelling that precedes the onset of chest pain and dyspnoea is classical for the diagnosis of PE.
Risk factors
Several conditions greatly increase the risk of blood clots. These are part of the standard VTE risk assessment in secondary care.
The major risk factor for development of PE is DVT. Symptomatic PE is associated with a DVT in up to 80% of cases, however, the DVT may only be symptomatic in a quarter (25%) of these people.
Major risk factors
- DVT
- Previous VTE
- Active cancer
- Recent surgery (e.g. within last 2-3 months)
- Significant immobility (e.g. hospitalisation, bed-rest)
- Lower limb trauma/fracture
- Pregnancy (+ 6 weeks postpartum)
Additional risk factors
- Combined oral contraceptive pill
- Long-distance sedentary travel (e.g. long-haul flights)
- Thrombophilia
- Obesity
- Others
Due to the high risk of VTE in patients admitted to secondary care (i.e. hospital), a VTE risk assessment should be completed on admission. This assesses whether a patient is suitable to receive prophylactic blood thinning medication (e.g. low molecular weight heparin) to reduce the risk of VTE.
Pathophysiology
The pathophysiology of PE is based on the principles of Virchow's triad.
The predominant theory for development of VTE is Virchow’s triad. This describes a triad of venous stasis, endothelial injury, and a hypercoagulable state. These are the three broad categories that contribute to the development of blood clots.
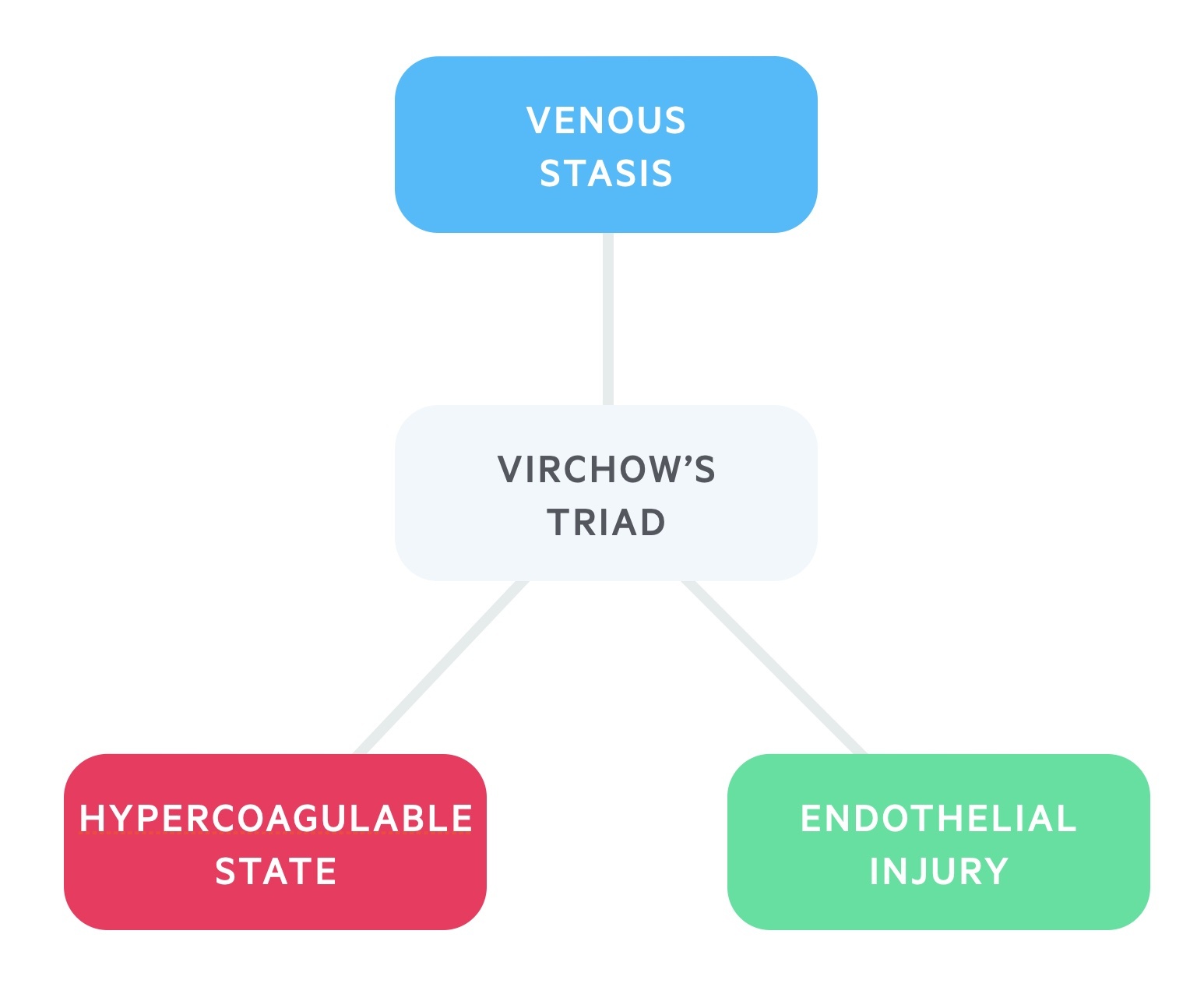
Patients may have inherited or acquired problems in one of more of these categories that leads to the development of blood clots. For example, a patient may have an inherited thrombophilia that increases the likelihood of clot formation or an active cancer which is associated with an acquired hypercoagulable state.
Natural history of PE
Occlusion of one or more of the pulmonary arteries leads to absence of perfusion to that area of the lung. There is said to be a ventilation/perfusion (V/Q) mismatch because ventilation is unaffected (i.e. the area of lung receives oxygen but not blood).
This V/Q mismatch may lead to hypoxia and clinical features of breathlessness. The area of lung may undergo infarction (death from abnormal blood supply), but is usually prevented by the bronchial circulation. The V/Q mismatch can lead to elevated pulmonary arterial pressure, alveolar collapse, worsening hypoxaemia and reduction in cardiac output.
Depending on the size and burden of emboli, the sudden increase in pulmonary arterial pressure may rise to a level the right ventricle cannot overcome. This can lead to hypotension, syncope and in more serious circumstances shock and/or death due to acute right ventricular failure.
Clinical features
PE is notoriously difficult to diagnose due to the variability in clinical presentation.
The classical presentation of PE is a patient with new onset shortness of breath (i.e. dyspnoea) with pleuritic chest pain (i.e. sharp pain on deep inspiration) with features of a DVT (unilateral, painful, erythematous leg swelling and venous distension).
Symptoms
- Dyspnoea (most significant symptom)
- Pleuritic chest pain
- Cough
- Haemoptysis
- Dizziness
- Syncope
- Leg pain and swelling
Signs
- Tachycardia (> 100 bpm)
- Low grade fever (> 37.5º)
- Hypoxia (sats < 94%)
Right heart failure
- Hypotension (BP < 90 mmHg or drop > 40 mmHg)
- Elevated JVP
- Tricuspid regurgitation (pansystolic murmur)
- Split second heart sound: elevated pulmonary pressure leads to delay in pulmonary valve closure.
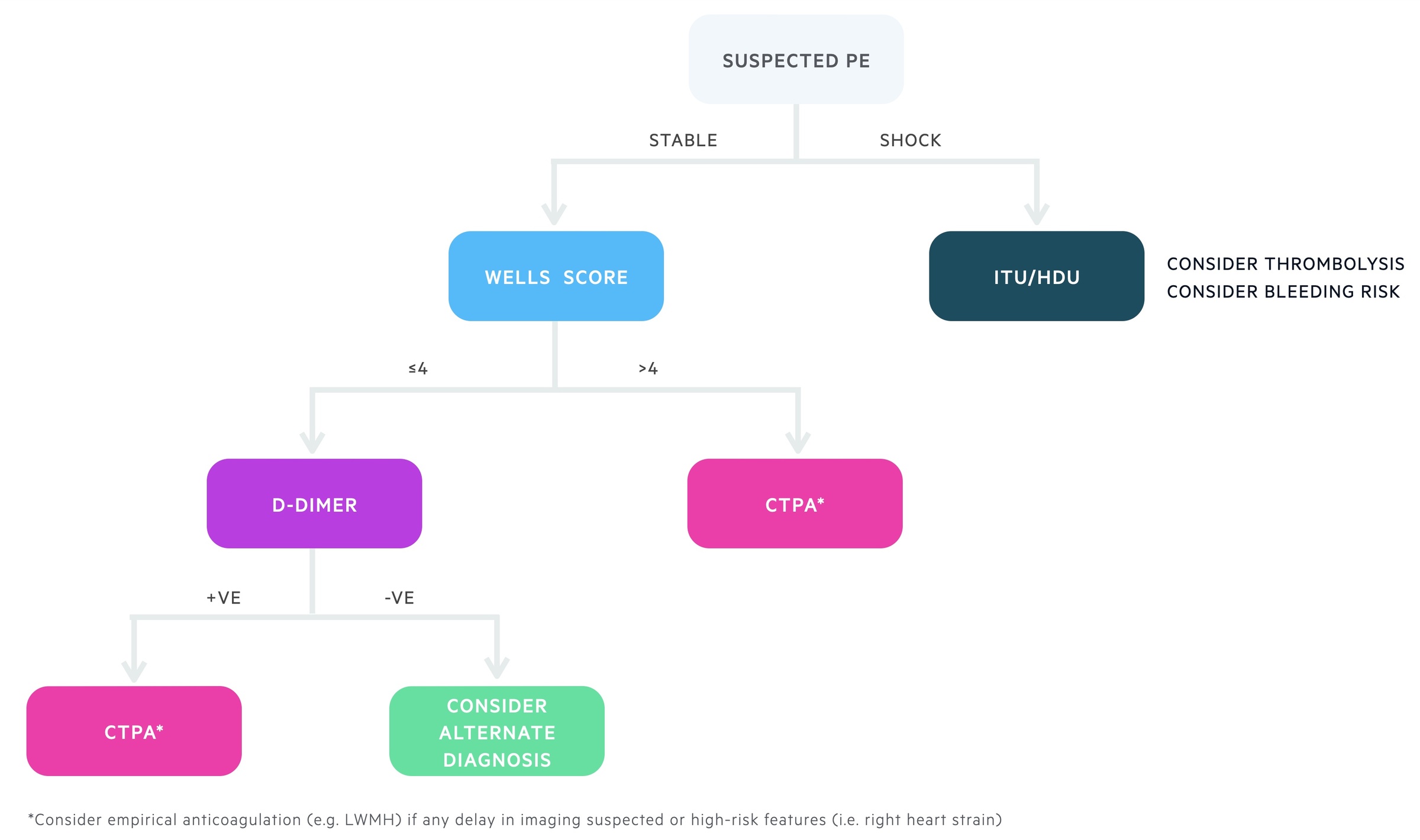
Wells score, d-dimer and PERC
The Wells score is used to assess the likelihood, or risk, of PE and guide further investigations.
Wells score
There are two different Wells' scores, one for the assessment of PE and one for DVT. In PE, a series of seven criteria are used that give a score between 0-12.5. This divides patients into low, moderate or high risk of PE.
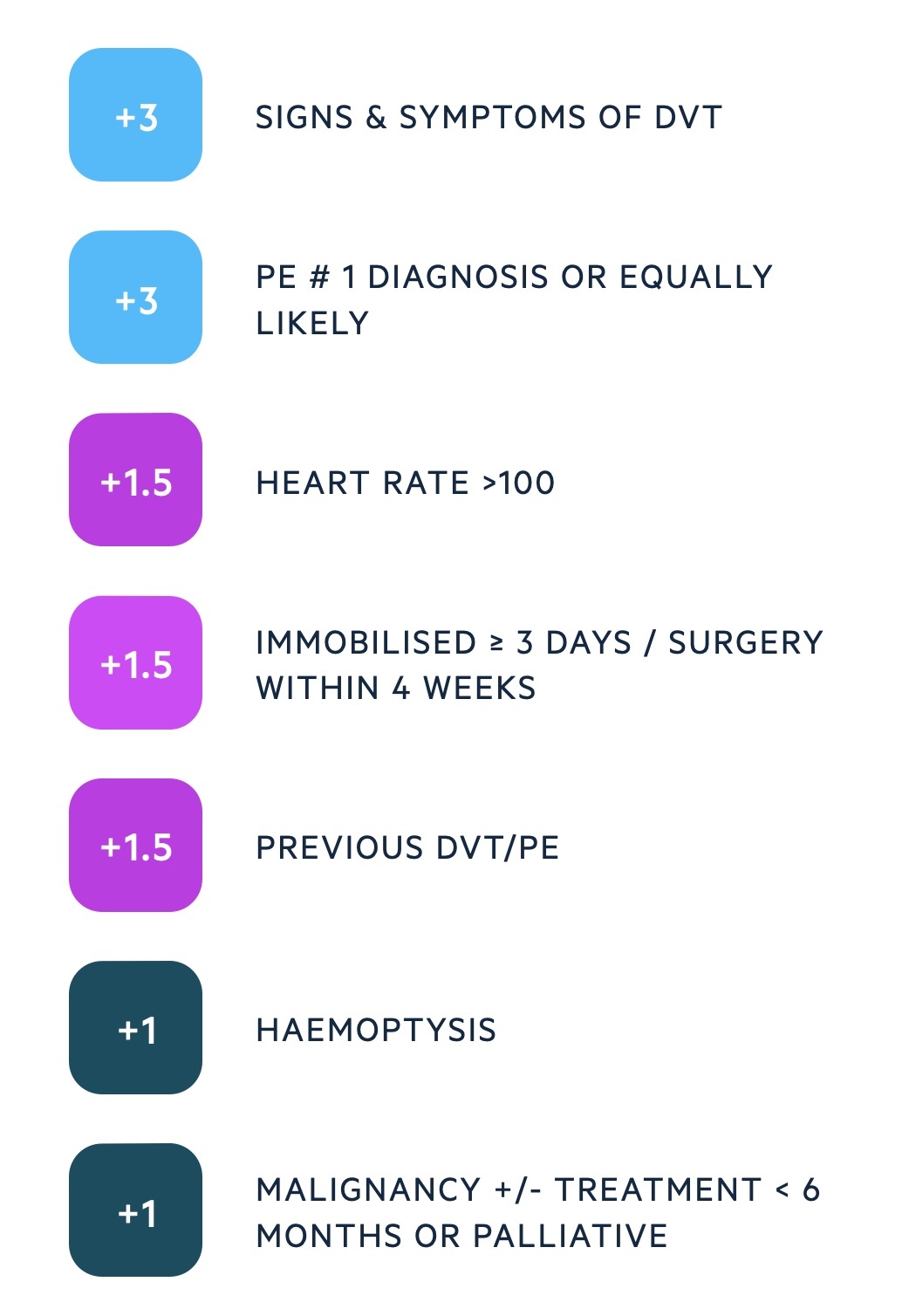
In the two-level model, patients are grouped into two categories:
- PE likely (score > 4): straight to computed tomography pulmonary angiography (CTPA), if not available immediately, interim anticoagulation if safe.
- PE unlikely (score ≤ 4): d-dimer blood test within four hours. If positive arrange CTPA. If negative, PE excluded consider alternative diagnosis.
D-dimer
D-dimer is a fibrin-degradation product, which is created when blood clots are broken down by the fibrinolytic system. It is a marker of VTE but can be raised in many conditions.
It is an excellent test at ‘ruling out’ PE due to its high sensitivity (~96%) and negative predictive value (~98%). However, it has a much lower specificity (40-60%), which means there are a lot of false positives. Therefore, it cannot be used to make the diagnosis.
PERC score
The d-dimer is an excellent screening test for PE, however, the high rate of false positives means an unnecessary number of patients may have a CTPA despite being low risk (pre-test probability <15%).
Consequently, the pulmonary embolism rule-out criteria (PERC score) is a useful emergency department tool that assesses whether a ‘low-risk’ patient should undergo further evaluation for PE with d-dimer. If none of the eight criteria are met, d-dimer or imaging is not required.
Diagnosis
The gold-standard investigation for the diagnosis of PE is a CTPA.
A CTPA is a type of CT scan that allows visualisation of the pulmonary arterial system with contrast. It should be used in patients with a high-probability of PE or those with a positive d-dimer who have been worked up for PE.
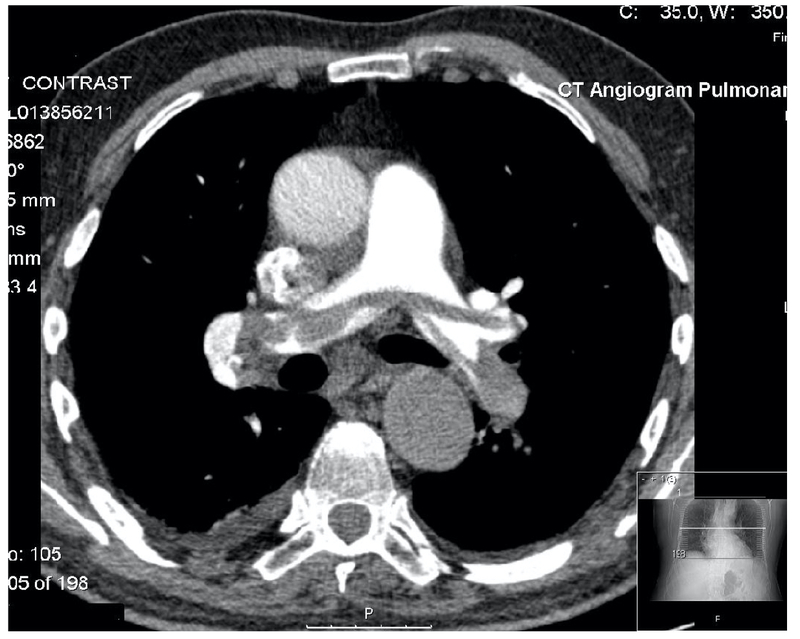
CTPA showing a large saddle embolus (grey opacification within the pulmonary arteries)
Investigations
ECG, echocardiogram and troponin are useful markers in the assessment of right ventricular strain/failure.
Regular observations and serial blood pressure monitoring is essential in any patient with suspected PE. This allows regular assessment for signs of ‘massive’ PE, which is characterised by haemodynamic instability.
ECG
- Common: sinus tachycardia, non-specific ST or T wave abnormalities
- Classical: S1Q3T3 pattern (deep S wave lead I, Q wave in lead III and T wave inversion in lead III)
- Right heart strain: right bundle branch block, ST depression and T wave inversion anteriorly (V1-V4) and/or inferiorly (II, III, aVF)
- Alternative diagnosis (e.g. acute coronary syndrome)
Bloods
- FBC
- U&E
- LFT
- Coagulation
- D-dimer
- Arterial blood gas: consider in equivocal cases
- Troponin: right heart strain
Imaging
- CXR: exclude any obvious alternative pathology that may explain symptoms
- CTPA
- V/Q scan: nuclear medicine scan that may be utilised in pregnancy or renal impairment
- Lower limb ultrasound: confirm presence of DVT
- ECHO: assessment of right ventricular strain/failure in patients with suspected ‘massive’ PE.
Acute management
The principle treatment for PE, or any thromboembolic disease, is anticoagulation.
Initial management of patients with a confirmed or suspected PE depends on their haemodynamic status and risk of further deterioration.
Any shocked patient (i.e. low blood pressure) should be moved into the resuscitation area of the emergency department or admitted to a high-dependency unit (HDU)/intensive treatment unit (ITU). Patients should be considered for thrombolysis therapy as per local guidelines.
The management of haemodynamically stable patients depends on their level of risk, which is dependent on their pulmonary embolism severity index (PESI) score and any objective evidence of right heart strain.
- Haemodynamically stable (high-risk): confirmed on CTPA, initiate anticoagulation. High PESI score or features of right heart strain, consider transfer to a higher level of care (HDU/ITU).
- Haemodynamically stable (low risk): confirmed on CTPA, initiate anticoagulation. Low PESI and no features of right heart strain, consider discharge with anticoagulation follow-up.
Anticoagulation
The are a numerous agents that can be used as anticoagulation in PE.
The choice of anticoagulation includes low molecular weight heparin (LMWH), direct oral-acting anticoagulants (DOAC), unfractionated heparin (UFH), and warfarin. Before confirmation of PE, LWMH is typically used as the empirical anticoagulant of choice.
Example choices of anticoagulation in PE (as per NICE 2020 guidelines - NG 158):
- Stable, no renal impairment or co-morbidities: offer apixaban/rivaroxaban. If not-suitable, LWMH for 5 days then offer edoxaban/warfarin*.
- Haemodynamic instability: consider thrombolysis and heparin-based anticoagulation (e.g. UFH) according to local guidelines. Treatment with heparins is not a contraindication to thrombolysis.
- Active cancer: consider DOAC (e.g. edoxaban). If not suitable, LMWH.
- Renal impairment: if creatinine clearance (CrCl) 15-50 ml/min, offer apixaban/rivaroxaban or LMWH for 5 days then edoxaban/warfarin*. If CrCl <15 ml/min offer LMWH/UFH as per local guidance.
*NOTE: If starting warfarin, must have two consecutive INR readings >2.0 before stopping LMWH (termed bridging therapy)
Thrombolysis
Thrombolysis refers to the use of recombinant tissue plasminogen activator (tPA), which is a fibrinolytic drug that breaks down clots. This is most commonly administered systemically (i.e. intravenous) but can be locally administered using a catheter device by an interventional radiologist.
Thrombolysis should be a senior-led decision and the patient should be in a high level of care (e.g. Resus/ITU). After excluding contraindications to thrombolysis, the indications include:
- Cardiac arrest with confirmed or suspected PE
- Confirmed PE with deterioration despite anticoagulation (i.e. worsening right ventricular strain, increasing oxygen requirements)
- Haemodynamic instability (BP < 90 mmHg for > 15 minutes), AND
- High clinical suspicious of PE
- Confirmed PE within 14 days
Long-term management
Patients are advised to continue anticoagulation for a minimum period of three months.
Beyond three months (3-6 months for active cancer), the duration of therapy depends on whether the PE was provoked or unprovoked, the location of the PE, bleeding risk and whether the underlying risk factor has been removed. Patients should be referred to a specialist anticoagulation clinic and the risk/benefit of continuing, stopping or changing therapy discussed.
In patients with an unprovoked PE, it is important to consider an underlying cause (e.g. inherited thrombophilia, cancer).
- Thrombophilia testing: looking for an inherited predisposition to form blood clots. Best completed by anticoagulation team*. Offered if coming off anticoagulation
- Cancer: clinical assessment and examination (including breast and testicular). Further investigations based on presence of cancer-specific clinical features (i.e. post-menopausal bleeding, breast lump).
*NOTE: Some anticoagulants can interfere with the tests carried out for thrombophilia so ideally need to be completed once off treatment.
PESI score
This is a prognostic scoring system that is useful to determine the appropriateness of outpatient versus inpatient management.
The score is composed of 11 variables. Summation of these gives a total score that categorises patients into 5 risk groups with an estimated 30-day mortality.
Those 'very low risk' or 'low risk' have a small overall risk of mortality and can be considered for outpatient management. The score should only be applied after PE has been confirmed on imaging. This is because patients may have a clinically significant PE despite low PESI score.
Complications
PE is still a major cause of morbidity and mortality.
PE with haemodynamic instability (i.e. Massive PE) is associated with high mortality (~20%) even in treated patients. PE is also one of the main direct causes of maternal death in pregnancy.
Long-term, PE may lead to chronic thromboembolic pulmonary hypertension (CTEPH) and associated right-sided heart failure. Factors associated with this complication are inadequate anticoagulation, large or residual thrombi and recurrent disease.
Last updated: September 2021
Have comments about these notes? Leave us feedback
