Chronic kidney disease
Notes
Overview
Chronic kidney disease (CKD) can be defined by the presence of kidney damage or reduced kidney function for three or more months.
Reduced kidney function is suggested by a reduction in the glomerular filtration rate (GFR) or by evidence of kidney damage which can be characterised by the presence of one or more of the following pathological markers:
- Albuminuria (e.g. albumin:creatinine ratio > 3 mg/mmol or > 30 mg/g)
- Urinary sediment abnormalities (e.g. white cell or red cell casts)
- Radiological abnormalities (e.g. polycystic kidneys)
- Pathological abnormalities (e.g. seen on renal biopsy)
- History of kidney transplantation
CKD is a common condition with a progressive nature. As CKD progresses towards end-stage renal disease (ESRD) it is associated with more symptoms, increasing complications, and the need for renal replacement therapy (RRT).
The inability of the kidneys to carry out their normal function can lead to problems with volume regulation, acid-base balance, calcium, and phosphate handling, and electrolyte abnormalities.
Classification
The classification of CKD is now based on two factors: chronicity and the precence of a reduced GFR or evidence of kidney damage.
The definition of CKD is the presence of either of the following for 3 months (i.e. chronicity) or more:
- Marker of kidney damage:
- Albuminuria (> 3 mg/mmol, i.e. A2/3)
- Abnormalities (including electrolyte derangement) secondary to tubular disorders
- Structural abnormalities
- Abnormalities on histology
- History of kidney transplant
- Reduced GFR: GFR < 60 ml/min/1.73 m2 (i.e. G3a-G5)
The GFR and ACR are two of the key measures in defining someone with CKD. The change towards using ACR in the classification of CKD reflects the increased risk of acute on chronic injury, end stage disease and all-cause mortality in patients with a high ACR. The Kidney Disease: Improving Global Outcomes (KDIGO) who are a not for profit organisation that help in the development of evidence-based renal guidelines, have defined a number of classes of GFR and ACR based on their measurements. These should be used to accurately stage a person with CKD.

CKD is increasingly common with advancing age and stages 3-5 affect up to 8.5% of the adult population. The higher the stage of CKD, the more frequent monitoring patients require. This helps to identify and manage complications and plan for Renal replacement therapy (RRT). The higher the ACR and lower the eGFR, the more at risk a patient is of adverse outcomes, which include CKD progression, acute-on-chronic kidney injury, all-cause mortality, and cardiovascular events.
Aetiology & pathophysiology
There are numerous causes of CKD, but the majority of cases are secondary to diabetes mellitus, hypertension and glomerulopathies.
Major causes include:
- Hypertensive nephropathy
- Diabetic nephropathy
- Glomerulopathies
- Inherited kidney disorders (e.g. PCKD)
- Ischaemic nephropathy (e.g. vascular disease)
- Obstructive uropathy
- Tubulointerstitial diseases
- Medications
Approximately 1 million nephrons are present in each kidney from birth. These nephrons contribute to the kidneys ability to maintain adequate glomerular filtration and allows the kidney to perform its normal functions (e.g. volume regulation, acid-base balance).
As we age there is a progressive loss in renal mass and a number of structural changes occur (e.g. glomerulosclerosis) leading to a decline in renal function. Following a peak in the third decade of life, there is an estimated annual decline of 1 mL/min/year in eGFR.
Regardless of the underlying cause, renal disease leads to progressive loss of nephrons and a subsequent reduction in the GFR. As the disease progresses, structural abnormalities may occur leading to kidney damage (e.g. albuminuria), and eventually, the kidneys start to lose their ability to carry out normal functions.
Clinical features
Patients are generally asymptomatic with CKD, but start to develop non-specific symptoms at more advanced stages (e.g. eGFR < 45ml/min).
It is always important to look for evidence of an underlying cause of CKD (e.g. large bilateral abdominal masses could be suggestive of PCKD).
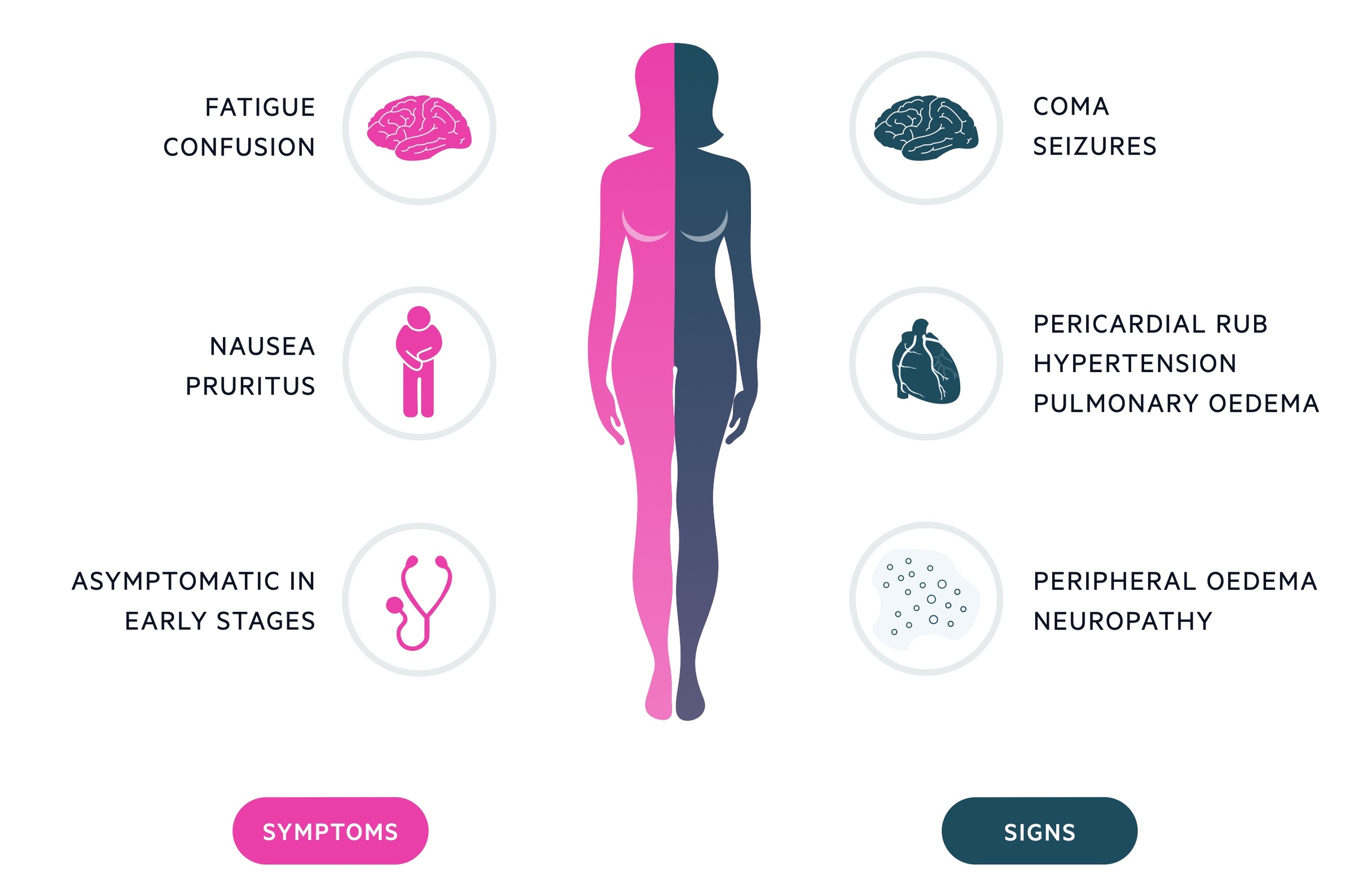
Symptoms
- Frequently asymptomatic in early stages
- Anorexia & nausea
- Fatigue & weakness
- Muscle cramps
- Pruritus
- Dyspnoea
- Oedema
Signs
- Pallor (secondary to anaemia)
- Hypertension
- Fluid overload (e.g. raised JVP, peripheral & pulmonary oedema)
- Skin pigmentation
- Excoriation marks
- Peripheral neuropathy
In severe renal impairment, patients may develop high urea levels that can lead to number of manifestations. These include uraemic encephalopathy (i.e. seizures, confusion, low consciousness), pericarditis (e.g. pericardial effusion and clinical rub on auscultation), and defective platelet function (causes bruising), amongst others.
Diagnosis
The diagnosis and subsequent monitoring of CKD is based on evidence of kidney damage and the measurement of the serum creatinine and urinary ACR.
There are a number of ways to calculate the eGFR from serum creatinine, all should be used with caution. Serum creatinine levels have a high individual variation changing with disease states, muscle mass, pregnancy and dietary intake.
Many laboratories will use the Modification of Diet in Renal Disease (MDRD) equation; however, NICE recommend the use the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for calculating the eGFR.
Indications for CKD testing include the following:
- Diabetes
- Hypertension
- Acute kidney injury
- Obesity with metabolic syndrome
- Cardiovascular disease
- Structural renal tract disease
- Proteinuria or persistent haematuria
- Family history
Depending on the results of the eGFR and ACR, patients can be classified into a particular stage of CKD. In patients with stable CKD (e.g. eGFR < 60ml/min without acute deterioration or ACR between 3 and 70 mg/mmol), it is important to repeat these tests within 3 months. Those with pronounced albuminuria (> 70mg/mmol) or significantly reduced eGFR (G4 or G5) require referral to a nephrologist.
Those with evidence of persistent haematuria in the absence of infection should be investigated for malignancy. NICE outline a specific set of referral criteria to a nephrologist that need to be taken into account when caring for patients with CKD, but that is beyond the scope of these notes.
Patient monitoring
The classification of CKD is a useful marker to determine how often to monitor renal function in a patient with CKD each year. For example, patients with G3a (eGFR 45-59) A1 (uACR < 3) should have renal function checked once a year. Conversely, a patient wit G4 (eGFR 15-29) A3 (uACR > 30) should have their renal function check atleast 3 times a year.
This is onlt rough guidance from NICE on the minimum amount of monitoring for patients with CKD. Some patients at high risk of progression or adverse outcomes (e.g. rapidly deteriorating renal function) may need their kidney function checked more frequently.
Investigations
Investigations may be used to help diagnose, monitor and assess for complications of CKD.
Urine
- Urine dipstick: if incidental proteinuria is found offer uACR and serum creatinine
- Urine microscopy
- ACR (spot test)
- ACR (24-hour collection)
- Electrophoresis (e.g. myeloma)
An urinary albumin:creatinine ratio (uACR) is used instead of a urinary protein:creatinine ratio (uPCR) because of its greater sensitivity at a lower level of proteinuria. If the uACR is significantly elevated (i.e. > 70 mg/mmol) then the uPCR may be used.
Bloods
- FBC
- U&Es (inc. eGFR)
- Bone profile
- PTH
- Bicarbonate
- LFTs
- Lipid profile
- Autoimmune screen (e.g. ANCA, ANA)
- Myeloma screen
Imaging
- Renal ultrasound
- Magnetic resonance angiography
- Echocardiogram
- ECG (high risk of CVS disease)
A renal ultrasound scan should be offered to patients with visible or persistent non-visible haematuria, evidence of obstructive uropathy, family history of PCKD, reduced eGFR (< 30ml/min) or accelerated progression of CKD.
Special
- Renal biopsy (useful in the identification of intrinsic causes of CKD)
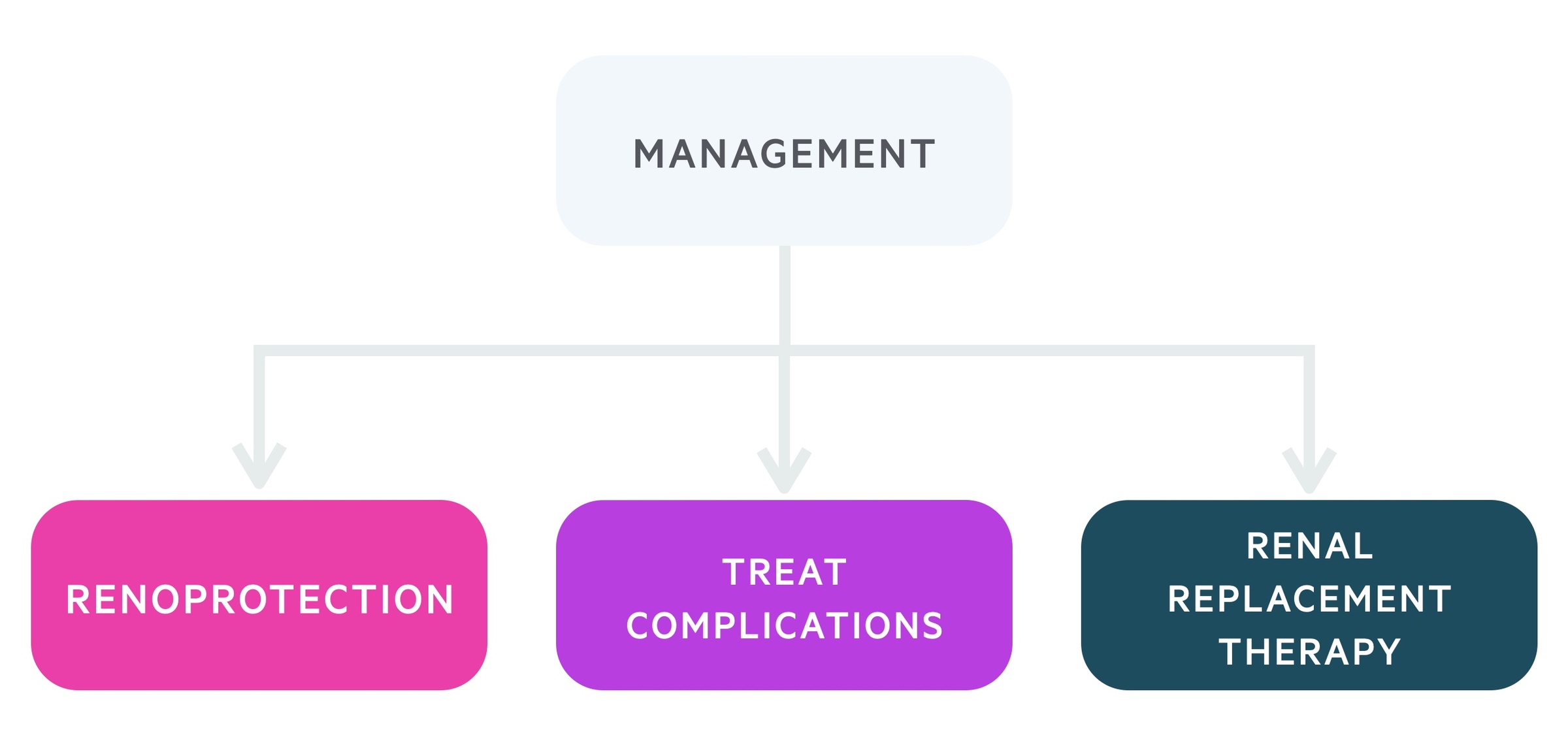
Management
The principles of CKD management are to treat the underlying cause, prevent or slow progression (e.g. renoprotective therapy), treat associated complications and plan for RRT.
Renoprotective therapy
Renoprotective therapy is aimed at slowing the progression of CKD, independent of the aetiology.
Renoprotective therapy is centered around blood pressure control and reducing proteinuria. Specific blood pressure targets have changed with the release of the new NICE chronic kidney disease guidelines.
A standard BP target of < 140/90 mmHg should be aimed for in patients with CKD. This is broadly in line with the NICE guidelines on hypertension. However, if the ACR is > 70 mg/mmol then a tigher blood pressure control may be targeted (< 130/80 mmHg).
In adults with CKD, hypertension and an ACR ≤ 30 mg/mmol, the advice on choice of anti-hypertensive should be line with the NICE guidelines on hypertension. In patients with more significant proteinuria, the ideal blood pressure agent is an ACE inhibitor or angiotensin receptor antagonist (both renin-angiotensin system antagonists). These drugs are both antihypertensive and antiproteinuric.
Offer a renin-angiotensin system antagonist in patients who are:
- Diabetic and have an ACR ≥ 3 mg/mmol (even in the absence of hypertension)
- Hypertensive and ACR > 30 mg/mmol
- ACR > 70mg/mmol (even in the absence of hypertension)
Before starting an ACE inhibitor or ARB patients need a baseline renal function assessed. The blood test should be repeated between 1-2 weeks after starting or any dose increase.
NOTE: do not routinely offer a renin–angiotensin system antagonist in CKD if the pretreatment potassium is > 5 mmol/L.
Sodium–glucose cotransporter 2 inhibitors
Dapagliflozin is a commonly used hypoglycaemic agent for patients with type 2 diabetes mellitus. The medication blocks SGLT2 receptors expressed on the proximal convoluted tubules of nephrons. In doing so, it prevents reabsorption of filtered glucose (i.e. its antidiabetic effect). However, inhibition has added benefits on renal haemodynamics including decreasing renal intraglomerular pressure and inhibiting the renin-angiotensin-aldosterone system. This means it can have beneficial effects for patients with chronic kidney disease and heart failure.
Dapagliflozin is now recommended in patients with CKD as an add on to patients who take an optimised dose of an ACE/ARB (unless contraindicated) and have:
- eGFR 25-75 ml/min/1.73m2, AND
- Type 2 diabetes mellitus, OR
- Urinary ACR ≥ 22.6 mg/mmol
Other therapies
- Statin therapy: prescribed in line with NICE recommendations for the use of statins
- Smoking cessation
- Antiplatelets for secondary prevention of CVS disease
Treating complications
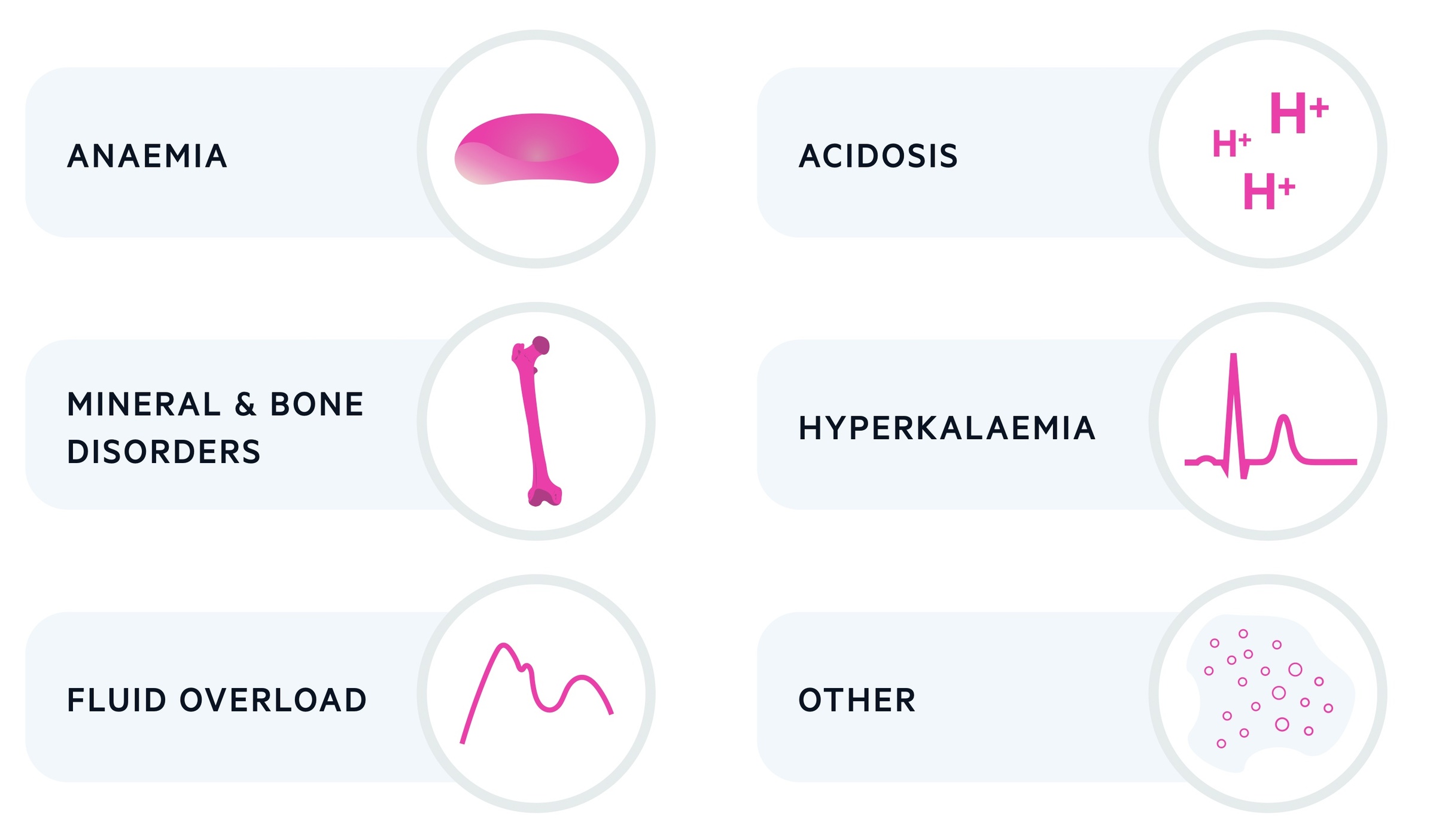
A number of important complications develop as a consequence of CKD, which include anaemia, hyperkalaemia, mineral and bone disorders, fluid overload and acidosis.
Anaemia
A normocytic normochromic anaemia is typical of CKD. This anaemia is normally multifactorial. A significant factor in advanced disease is a reduction in the production of erythropoietin (EPO), the hormone that drives erythropoiesis.
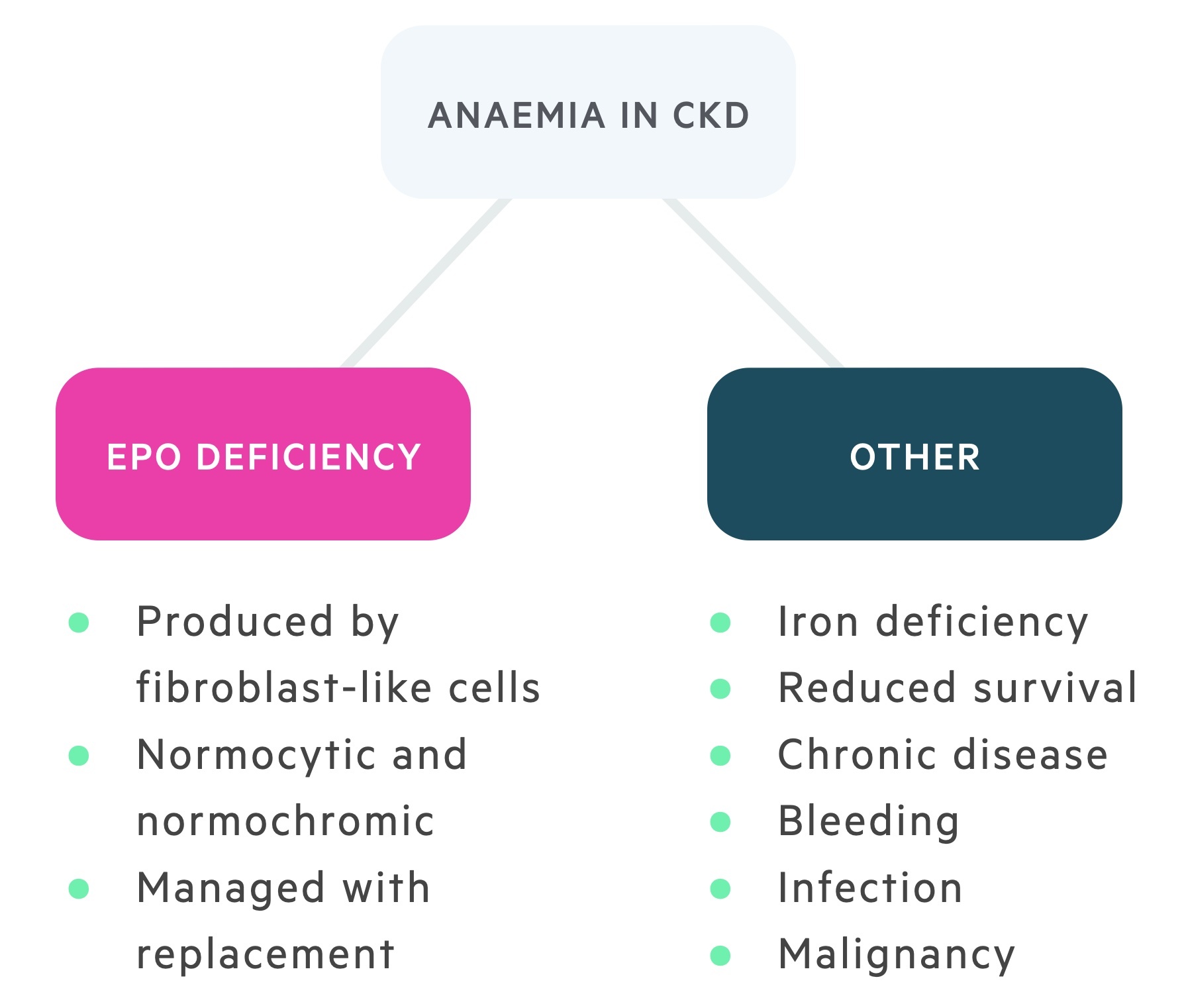
It is still important to assess patients for other potential causes of anaemia (e.g. iron-deficiency, folate deficiency), which can subsequently be corrected. The main management for anaemia in CKD is the use of erythropoietin-stimulating agents (ESA) such as epoetin alfa. In order for EPO therapy to be effective, patients need to have adequate stores of iron that should be regularly checked and replaced. In fact, initiation of EPO is not recommended until iron-deficiency has been managed.
Blood transfusions should be avoided where possible in patients who are being considered for renal transplantation. This is because of the risk of sensitisation (i.e. development of antibodies) to human leucocyte antigens (HLA).
The aspirational haemoglobin concentration in patients with CKD-associated anaemia is 100-120 g/L.
Hyperkalaemia
The ability of the kidneys to maintain adequate acid-base homeostasis and electrolyte balance diminishes with worsening renal function. Many medications, including NSAIDs and potassium-sparing diuretics, may worsen hyperkalaemia. Furthermore, uncontrolled metabolic acidosis may also worsen potassium handling.
Acute rises in potassium should be managed as a medical emergency. This involves stabilisation of the myocardium (with calcium gluconate) and driving potassium into the intracellular compartment (with insulin/dextrose). Chronic elevations in serum potassium can be managed with low potassium diets, potassium-binding resins and correction of acidosis. In fact, the use of newer potassium-binding resins (e.g. Patiromer, sodium zirconium cyclosilicate) may help to lower potassium levels and facilitate use of renoprotective drugs through their action of binding potassium ions within the gastrointestinal tract. This then allows the use of drugs such as ACEi and ARBs that would otherwise be contraindicated with high levels of potassium.
See our hyperkalaemia notes for more detail.
Mineral and bone disorders
In CKD, disorders of mineral and bone metabolism reflect a complex spectrum of pathology that results from abnormal calcium and phosphate handling. In health, the kidneys have an important role in the maintenance of calcium homeostasis. They are able to activate vitamin D (calcitriol), a fat-soluble vitamin important for absorption of calcium from the gastrointestinal tract. They are also involved in the reabsorption of calcium and excretion of phosphate.
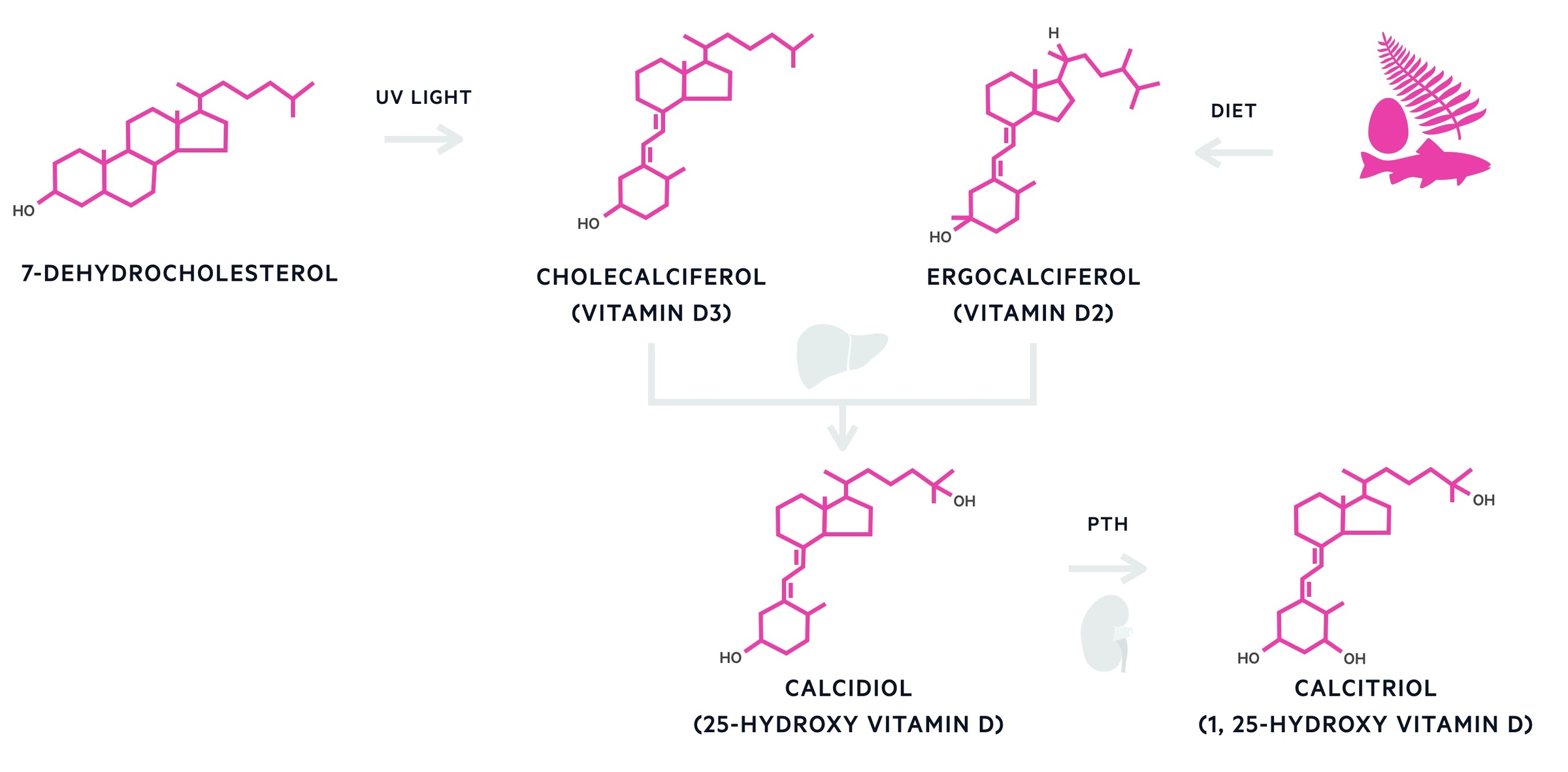
In disease, reduced kidney function (usually associated with a GFR < 30ml/min) leads to hypocalcaemia, hyperphosphataemia and hyperparathyroidism (secondary hyperparathyroidism). These biochemical abnormalities may then lead to boney pathology (e.g. adynamic bone disease, osteomalacia, osteoporosis and osteitis fibrosa cystica). The term ‘renal osteodystrophy’ is used exclusively for this type of bone pathology seen in CKD.
The management of mineral and bone disorders requires management of the underlying biochemical abnormalities.
- Hypocalcaemia: dietary supplements and calcitriol.
- Hyperphosphataemia: dietary restriction and phosphate binders (e.g. calcium acetate).
- Hyperparathyroidism: calcimimetics or surgery.
Fluid overload
In the presence of significantly reduced GFR, the kidneys are unable to adequately controlled fluid volume. This leads to hypervolaemia and patients may have evidence of peripheral oedema, ascites, raised JVP, gallop rhythm and bilateral pleural effusions.
Fluid overload can be managed with a combination of fluid restriction, reduced sodium intake and the use of oral diuretics (e.g. furosemide).
Acidosis
Patients with CKD have an increased tendency to retain hydrogen ions because of abnormalities in their acid-base homeostasis. Acidosis may also exacerbate hyperkalaemia, further compounding the metabolic abnormalities seen in CKD.
Acidosis is characterised by a low pH and low bicarbonate levels. Oral sodium bicarbonate therapy may be considered in patients with an eGFR < 30 ml/min/1.73m2 and bicarbonate level < 20 mmol/L.
Renal replacement therapy
Haemodialysis, peritoneal dialysis and renal transplant are all forms of RRT that are indicated for ESRD.
In-depth analysis of the types of RRT, their indications, contraindications and efficacy are beyond the scope of these notes. However, we will briefly cover some of the key aspects of each type.
Haemodialysis
Haemodialysis involves the removal of waste products and other substances by passing blood through a dialysis machine. Blood comes into contact with a semi-permeable membrane, which contains the dialysate on the other side. Substances may then diffuse between the two fluids (blood and dialysate).
Haemodialysis requires intravenous access in the form of an arteriovenous fistula or an artificial line (e.g. portacath). Patients usually have haemodialysis 3-4 times a week for several hours.
Peritoneal dialysis
Peritoneal dialysis is achieved by using the peritoneal cavity as the primary site of ultrafiltration. A catheter (e.g. Tenckhoff catheter) is inserted into the abdominal cavity, which allows the infusion of the dialysate. The dialysate then dwells within the abdomen using the peritoneum as a semi-permeable membrane for the transfer of waste products. The dialysate can then be removed after a certain amount of time and the procedure repeated.
In general, peritoneal dialysis can be achieved by continuous ambulatory peritoneal dialysis (CAPD) where multiple exchanges are made each day, or by automated peritoneal dialysis (APD) where the exchange is made overnight when the patient sleeps.
Renal transplant
Renal transplant is considered the gold-standard for RRT. Transplantation may be from living donors or non-living donors as long as there is MHC compatibility, which may mediate graft rejection. Non-living donors include donors after cardiac death (DCD) and donors after brain death (DBD).
Renal transplantation requires the use of long-term immunosuppressive therapy to stop the recipient's immune system from ‘attacking’ the donor tissue.
Despite the overt success of transplantation, it can be associated with a number of complications including graft rejection, complications from immunosuppressive agents (e.g. malignancy, infection) and disease recurrence.
Last updated: December 2022
Have comments about these notes? Leave us feedback