Subarachnoid haemorrhage
Notes
Definition
Subarachnoid haemorrhage (SAH) is bleeding in the subarachnoid space between the arachnoid and pia mater meningeal layers.
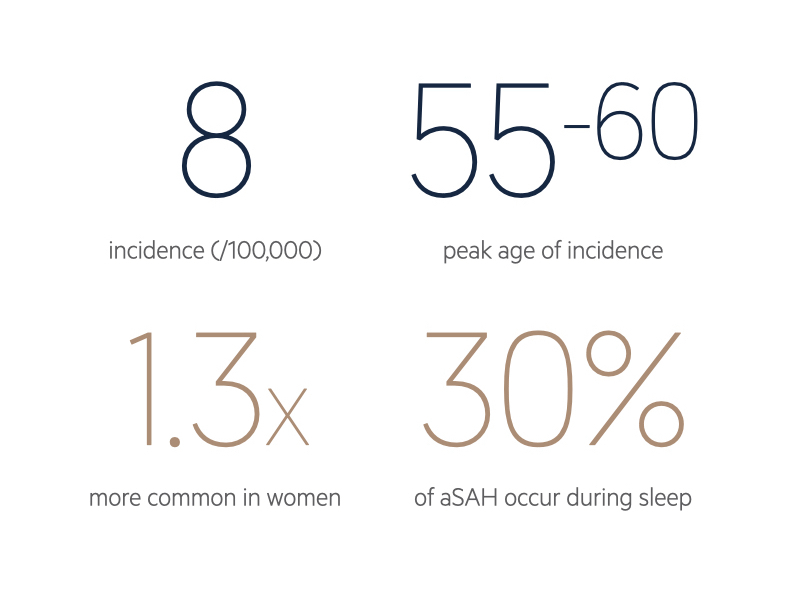
Aetiology
SAH can be a life-threatening emergency and it is estimated that 10-15% of patients die before they reach hospital.
SAH can be divided into traumatic or spontaneous
- Traumatic (tSAH): most common cause of SAH. Usually in setting of a head injury (e.g. fall, assault, road traffic collision)
- Spontaneous: commonly due to rupture of a cerebral aneurysm (aSAH)
Traumatic
Trauma is the most common cause of SAH. There is usually evidence of trauma in the clinical history (e.g. road traffic accident). An isolated SAH in the setting of mild traumatic brain injury usually has a good prognosis. Subarachnoid haemorrhage in the context of severe traumatic brain injury is usually associated with other forms of injury such as intracerebral haemorrhage or extradural haemorrhage.
Spontaneous
The most common causes of a spontaneous SAH are:
- Rupture of a cerebral aneurysm (75 to 80%)
- Arteriovenous malformation (5%)
- Rarer causes: including arterial dissection, vasculitis, tumour, drugs (e.g. cocaine), sickle cell disease
Risk factors
The primary risk factors associated with a spontaneous SAH are smoking and hypertension.
Risk factors for spontaneous SAH can be divided into modifiable and non-modifiable.
Modifiable
- Hypertension
- Smoking
- Alcohol abuse
- Substance misuse
Non-modifiable
- Sex: higher incidence in female
- Race: higher incidence in Japanese/Finnish populations
- Family history of aneurysms
- Genetic predisposition: seen in conditions such as autosomal dominant polycystic kidney disease (increased tendency to form berry aneurysms) or type IV Ehlers-Danlos syndrome
Anatomy
The brain is encased in three meningeal layers: the dura, arachnoid and pia mater.
Three components of neuroanatomy are critical understand SAH:
- Meninges
- Circle of Willis
- Aneurysm location
Meninges
The brain is encased in three meningeal layers, which are the dura, arachnoid and pia mater (outside to inside). The pia mater is adherent to the surface of the brain and spinal cord. The subarachnoid space is found between the arachnoid and pia mater. There are multiple bloods vessels that run in this space including the circle of Willis.
Circle of Willis
The circle of Willis is an arterial polygon and is the main arterial blood supply to the brain. It connects the anterior and posterior cerebral circulation, which arise from the carotid and vertebrobasilar systems, respectively.
- Carotid system: the common carotid artery gives off the internal and external carotid arteries. The internal carotid artery is vital to the circle of Willis. It gives off the anterior and middle cerebral arteries as well as the posterior communicating artery.
- Vertebrobasilar system: the two vertebral arteries join to form the basilar artery. Multiple branches arise from this artery. The basilar artery gives off the posterior cerebral arteries and joins anterior circulation via the posterior communicating artery.
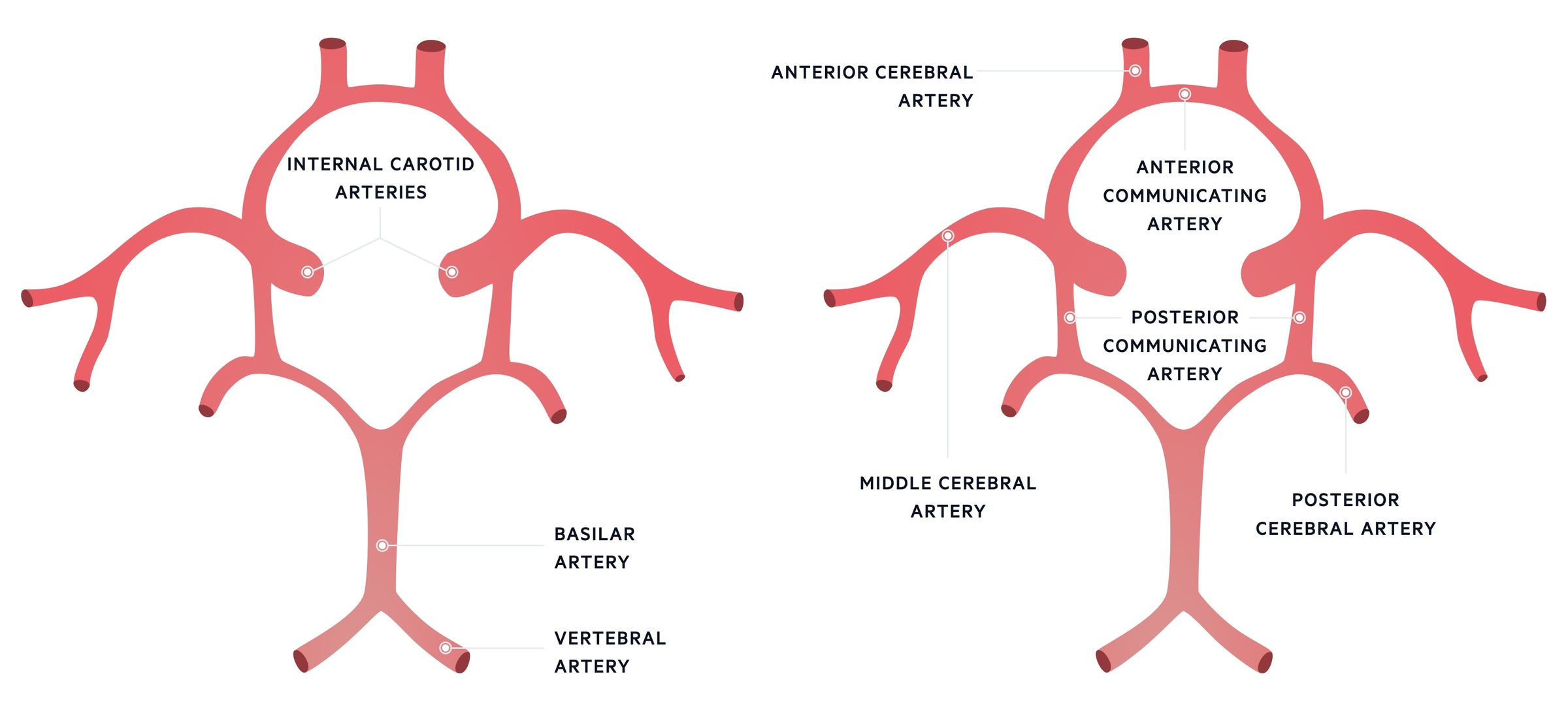
Circle of Willis
Aneurysm location
Saccular or berry aneurysms are usually located at the branching points of major blood vessels. The maximum haemodynamic stress in a vessel is seen at these points. Saccular aneurysms tend to occur in the anterior communicating artery (30-40%), posterior communicating artery (25%) and middle cerebral artery (20%). Approximately 10% of aneurysms occur at basilar tip or the bifurcation.
Aneurysms occur all along the anterior and posterior circulation.
Clinical features
Patient classically complain of a 'thunderclap' or sudden-onset headache.
Spontaneous SAH is associated with a sudden-onset or 'thunderclap' headache. This describes a severe headache that reaches maximum intensity within seconds (‘the worst headache of my life’).
Headache may be associated with photophobia (difficulty tolerating light) and neck stiffness. Collectively, these three features are known as meningism.
Symptoms
- Headache (97% of cases)
- Photophobia
- Neck stiffness
- Nausea
- Vomiting
Signs
- Neck stiffness
- Cranial nerve palsy (e.g. third nerve palsy)
- Reduced consciousness (coma)
- Diplopia (double vision)
- Ptosis
Classic signs of meningism
Both Kernig’s and Brudzinski’s are classical signs of meningeal irritation. May be seen in bacterial meningitis or subarachnoid haemorrhage. However, absence of these signs does not exclude these diagnoses.
- Kernig's sign: inability to fully extend at the knee when the hip is flexed at 90º due to pain
- Brudzinski's sign: spontaneous flexion of the knees and hips on active flexion of the neck due to pain
Third nerve palsy
A subarachnoid haemorrhage or enlarging cerebral aneurysm may be associated with a third nerve palsy.
The third cranial nerve is known as the oculomotor nerve. It has important motor function to the pupil, eyelid and extraocular muscles. A third nerve palsy can occur anywhere along its path from nucleus to orbital apex.
An isolated acute third nerve palsy is concerning for an enlarging aneurysm with risk of rupture and requires urgent assessment. The patient may complain of double vision and the eye will appear 'down and out' with ptosis (drooping eyelid), pupillary dilatation (if pupil involved) and loss of the light reflex (if pupil involved).
The presence or absence of pupillary involvement helps to determine the aetiology. This is because parasympathetic motor fibres associated with the pupillary light reflex are situated superficially within the nerve. Therefore, compressive lesions (e.g. aneurysms) will affect fibres leading to pupillary dilatation and loss of light reflex. On the other hand, vascular lesions (e.g. ischaemia, diabetes mellitus) tend to affect the inner fibres preferentially. This 'spares' the superficial parasympathetic fibres leading to pupillary sparing.
For more information see Third nerve palsy notes.
Diagnosis
Diagnosis of spontaneous SAH is suspected clinically and then confirmed on imaging or cerebrospinal fluid analysis.
SAH should be suspected in any patient with a sudden or rapid onset severe headache, which is classical of SAH. These patients require urgent assessment and computed tomography (CT) of the head.
Imaging
A plain CT Head is required to check for the presence of acute blood in the subarachnoid space. If performed within six hours of onset of symptoms (ictus), it has a sensitivity of 98.7% and specificity of 99.9% for detecting a subarachnoid haemorrhage. Additional imaging is required following diagnosis (discussed below).
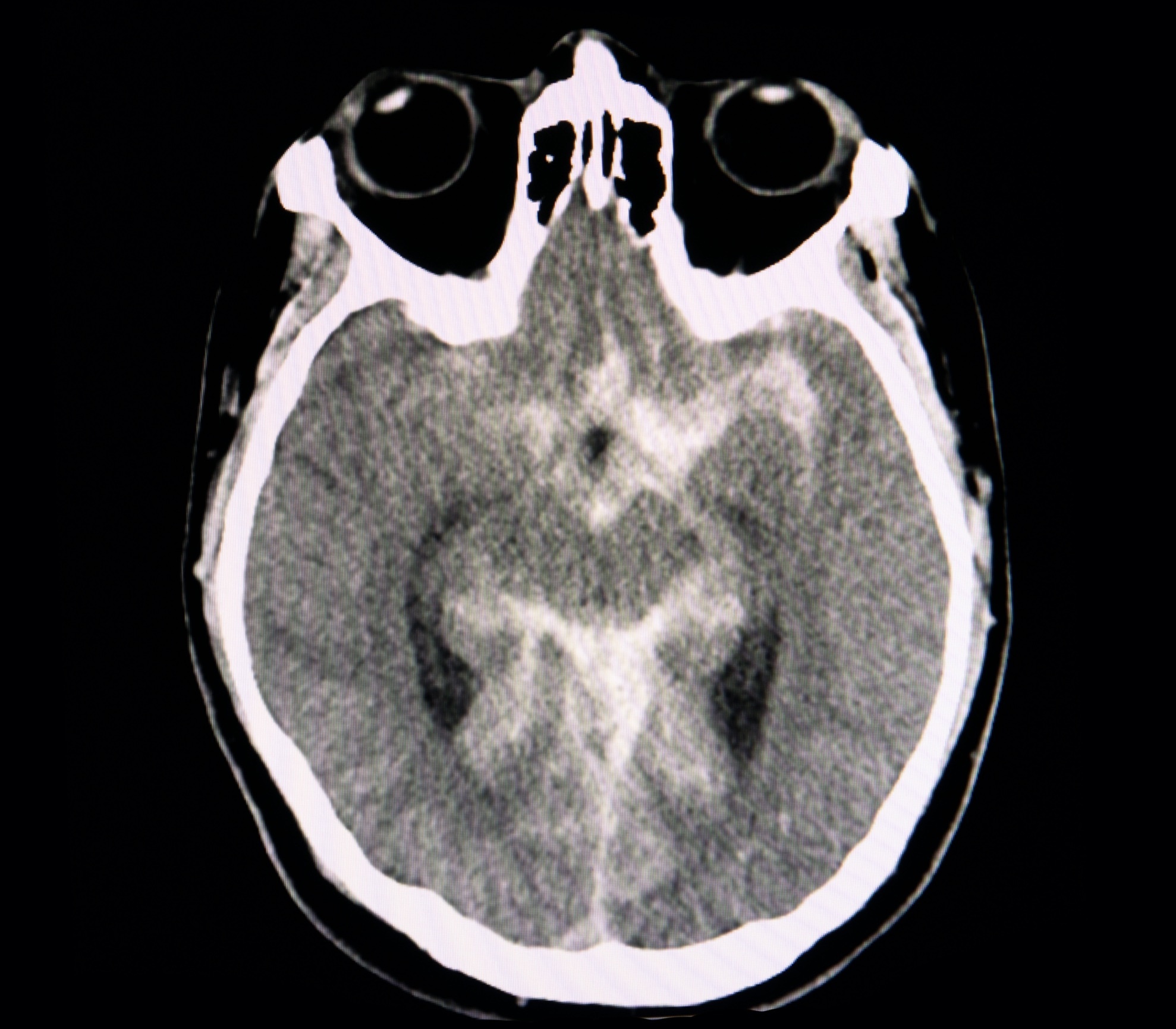
CT head showing subarachnoid haemorrhage
This high sensitivity and specificity declines over time. The sensitivity is only 58% at day 5. If CT is performed > 6 hours from symptom onset, then a lumbar puncture is required to exclude the diagnosis.
If a CT is performed within 6 hours of symptom onset and does not show a SAH, the risk of aneurysmal SAH is < 1%. A meta-analysis in 2016 showed that the risk of 'missed SAH' was < 1.5 in 1000 patients with SAH if no lumbar puncture was completed in patients meeting the following criteria:
- Presentation: isolated 'thunderclap' headache
- Timing: normal CT head <6 hours of headache onset
- Interpretation: CT interpreted by an experienced radiologist
- Clinical examination: Normal neurological examination
Therefore, the need for lumbar puncture should be a risk/benefit decision based on history, neurological status, and local pathways in patients presenting and being scanned within 6 hours. Current NICE guidelines published in November 2022 (NG228) advise that a lumbar puncture does not routinely need to be completed in this scenario.
Cerebrospinal fluid analysis
If a CT Head does not show any evidence of SAH then a lumbar puncture (LP) is required.
A LP is used to collect and assess the cerebrospinal fluid (CSF) for evidence of SAH. This is particularly important for patients presenting > 6 hours from onset.
Typically, haemoglobin and bilirubin are not found in CSF but after a SAH, red blood cells lyse and release oxyhaemoglobin which is converted to bilirubin. Oxyhaemoglobin on its own suggests a recent bleed or a traumatic lumbar puncture but the presence of both oxyhaemoglobin and bilirubin is suggestive of SAH. The LP should be delayed by 12 hours from symptom onset to improve the sensitivity of detecting red blood cell breakdown products (e.g. xanthochromia/bilirubin)
Four factors are important when completing a LP for SAH:
- Opening pressure: elevated in SAH
- Red cell count: elevated in SAH. May be seen in a traumatic LP. Sequential tubes (e.g. 1-4) can be taken to look for a fall in red blood cell concentration. A traumatic LP is supported by a significant reduction in red cells between the first and last tubes.
- Xanthochromia (visual inspection): a breakdown product of haemoglobin. May be detected visually. At 12 hours post-SAH, 100% of patients will have xanthochromia. Lasts for ~2 weeks.
- Bilirubin (spectrophotometry): spectrophotometry detects blood breakdown products, in particular, bilirubin. More sensitive than visual inspection. Samples must be protected from light and sent immediately to the laboratory for analysis. Lasts for ~4 weeks (if extensive bleed).
Further imaging
Once SAH has been established, angiography is needed to determine if there is any underlying vascular lesion. This involves a contrast scan called a CT Angiogram (intracranial). This helps to determine if there is any underlying aneurysm or arteriovenous malformation. Some aneurysms may go undetected on a CT angiogram, especially if the quality is poor.
The gold standard is digital subtraction angiogram (DSA). This is an invasive procedure which requires an arterial puncture (femoral or radial) and insertion of a guidewire and contrast. This procedure is both diagnostic and therapeutic as aneurysms can be investigated but also secured to prevent a re-bleed using coils and other various interventional devices.
Unruptured aneurysms
It is very important to distinguish unruptured intracranial aneurysms (UIA) from aSAH.
UIA and aSAH are two separate entities (UIA ≠ aSAH). With the increasing use of imaging, more and more incidental aneurysms are being detected. Spontaneous SAH due to a ruptured aneurysm is a clinical emergency, whereas UIA are managed in a multi-disciplinary neurovascular setting and in outpatient clinics. There are scoring systems available to help manage UIAs including PHASES and ISUIA.
Investigations
Patients with a suspected SAH require urgent observations, bloods, imaging +/- lumbar puncture.
Bedside
- Observations
- ECG: a variety of ECG changes may occur in SAH including QTc prolongation, bradycardia (Cushing's reflex), T wave inversion or ST elevation/depression. The latter is associated with a stunned myocardium and may mimic acute coronary syndrome.
NOTE: Cushing's reflex is a physiological response to raised intracranial pressure, which causes hypertension, bradycardia and irregular respirations (Cushing's triad). It is a late sign and suggests impending brainstem herniation.
Bloods
- FBC: check for anaemia and thrombocytopenia
- U&E: check the electrolytes, especially sodium
- LFT: disorder or history of alcohol abuse
- Bone Profile: check for calcium
- Coagulation: check for clotting disorder
- Group & save: if going for surgery
In the acute setting, additional bloods such as CRP may be performed if the differential for a headache includes an infective cause such as meningitis.
Imaging
- CT head
- CT Angiogram (intracranial)
Discussed further in diagnosis.
Special tests
- Lumbar puncture
Discussed further in diagnosis.
Management
Subarachnoid haemorrhage is a medical emergency that requires urgent management.
The management of SAH has several key elements.
- Initial management: refers to the immediate management in the aftermath of a bleed. Also involves further investigations into the bleed.
- Medical management: series of management strategies to monitor the patient closely and prevent further injury.
- Surgical management: placement of an external ventricular drain may be required if complications develop (e.g. hydrocephalus)
- Aneurysm management: refers to strategies for management of an aSAH.
Whilst the initial investigative work-up and management happens under emergency or medical care, spontaneous aSAH is managed under neurosurgery.
Initial management
Patients with a SAH can present fully alert or conscious or in a coma. This is assessed using the Glasgow Coma Score (from 3 to 15) and determines the grading (see under clinical scores). A systematic ABCDE is utilised in emergency settings. If the patient has a low GCS (below 8) they will require airway management. Otherwise, they are investigated with appropriate imaging or lumbar puncture as needed.
Medical management
A patient presenting with a suspected aSAH should be monitored closely with neuro-observations.
As there is always a risk of re-rupture, patients should be given analgesia, anti-emetics to prevent Valsalva whilst vomiting. Intravenous fluids are commenced with strict input/output monitoring. As patients require close monitoring they are transferred to neurosurgery to a level 1 bed or high dependency bed (HDU).
Patients are given nimodipine 60mg every 4 hours for 21 days from ictus of symptoms. It is incorrectly stated this reduces the risk of cerebral vasospasm. This is partially the case as the trial this is derived from demonstrated more favourable outcomes and reduced incidence of vasospasm in the group that received nimodipine compared to the control.
Most importantly, they require a prompt CT angiogram. If there is indeed an aneurysm, they will be transferred to the neurosurgical team for further management
Summary:
- Neuro-observations
- Anti-emetics & analgesia
- Fluid balance monitoring: consider IV fluids
- Electrolyte correction
- Calcium channel blockers (Nimodipine): guided by neurosurgeons
- CT angiogram
Surgical management
If the patient presents with a low GCS in the setting of SAH, they may have developed a neurosurgical emergency known as hydrocephalus (accumulation of CSF in the ventricular system) which can be diagnosed on a CT Head. Other causes of low GCS may occur including seizures.
After initial resuscitation and airway management, the presence of hydrocephalus may require insertion of an external ventricular drain (EVD). This is a form of CSF diversion and helps to reduce intracranial pressure. This does not drain the blood from a SAH nor is that the purpose of this procedure. It is intended to manage hydrocephalus
Aneurysm management
If an aneurysm is detected, it needs to be secured to prevent a further bleed. This is a complex decision made under the neurosurgical team but can be done under interventional radiology during a DSA.
The two principle methods include:
- Coiling: completed during DSA by interventional radiology team. Aneurysm is filled with platinum coils that induce clotting (embolisation) and this closes off the sac. Typically a general anaesthetic is used.
- Clipping: completed by neurosurgical team via a craniotomy. Patients receive a general anaesthetic and undergo a craniotomy and microscopic assisted clipping of the aneurysmal neck.
Either method will secure the aneurysm and minimise the risk of further bleeds. Coiling is more commonly utilised.
Complications
SAH are associated with several complications including rebleeding and vasospasm.
Re-bleeding
If an aneurysm has been identified, it is prone to re-bleeding until it is secure. This requires coiling or clipping.
Vasospasm
There is a risk of cerebral vasospasm in SAH as the blood load can irritate the arterial vasculature prompting a spasm which may cause strokes.
Vasospasm is a major complication from day 3-7 post ictus. Patients receive nimodipine for 21 days from ictus to reduce poor outcomes.
Hydrocephalus
Early or late hydrocephalus may develop post SAH which requires CSF diversion via an EVD or a lumbar drain.
Other
- Syndrome of inappropriate ADH (SIADH) secretion
- Cerebral salt wasting syndrome: excess urinary excretion of sodium (leads to hyponatraemia)
- Infections: chest/urine
- DVT (immobilisation, co-morbidities, VTE prophylaxis contraindicated)
- PE (immobilisation, co-morbidities, VTE prophylaxis contraindicated)
- Cardiovascular
Clinical scores
Several scores associated with SAH, including the modified World Federation of Neurological Societies (mWFNS) and modified fisher.
Modified World Federation of Neurological Societies
This score is used for grading the severity of an aneurysmal SAH and the modified Fisher for the risk of vasospasm associated with SAH.
The mWFNS is based on the Glasgow Coma Score (3-15) of the patient on presentation with an aSAH. This score is linked to likely outcomes of the patient using the Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS).
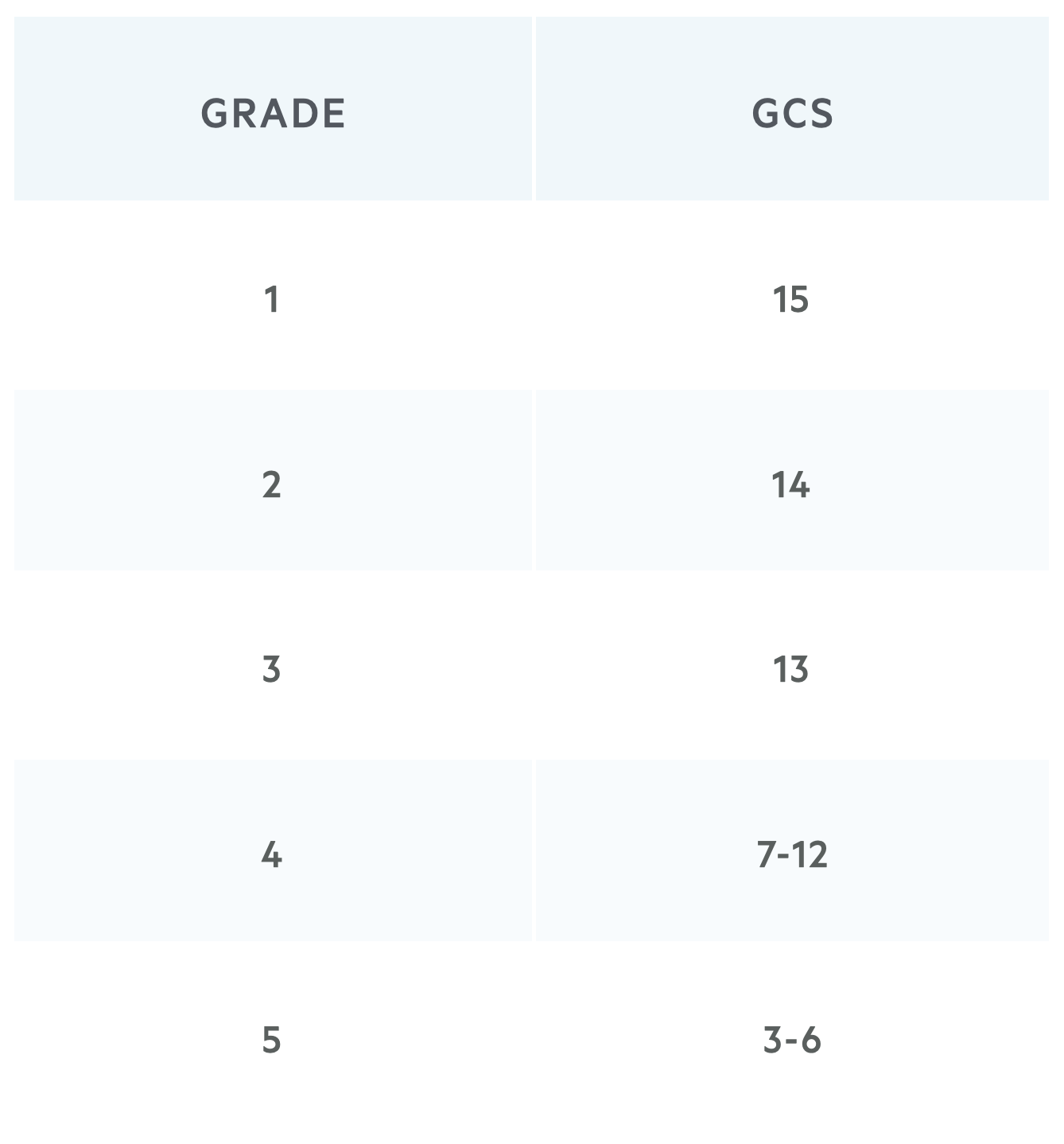
For example, a patient with a proven SAH on CT Head who is GCS E4V4M6 (14) would be classed as mWFNS Grade 2. Please note, some older resources refer to the original WFNS grading system from 1988 which mentions neurological deficit and this is now considered outdated. The most recent version uses only GCS.
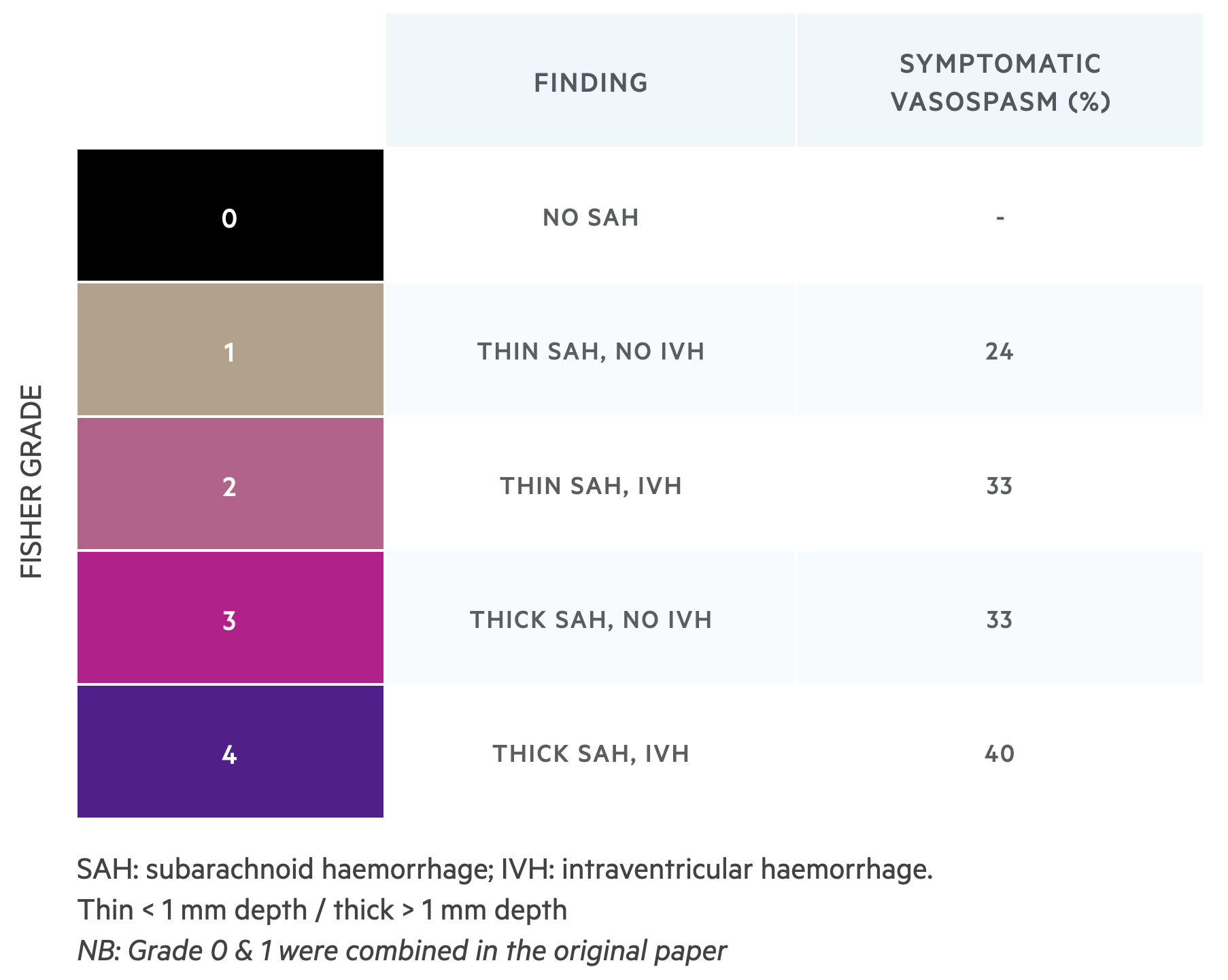
Modified Fisher
This score is used to assess the risk of vasospasm associated with SAH.
The modified Fisher scale is used to determine the risk of vasospasm based on the findings on imaging. In any bleed, vasospasm can occur due to contact between blood products and the lining of the arterial wall. This can result in strokes and delayed ischaemia. The amount and degree of blood load can determine the risk of vasospasm.
Last updated: July 2021
Have comments about these notes? Leave us feedback
